
(a)
Interpretation:
The CN stretching mode that absorbs the higher-frequency IR photons is to be indicated for given pair of compounds. The reason for it is to be explained.
Concept introduction:
We simplify the picture of molecular vibrations by considering the ball-and-spring model, which treats bonds as simple springs that connect atoms together. According to Hooke’s law, the spring vibrates at a particular frequency (
Answer to Problem 15.19P
The CN stretching mode that absorbs the higher-frequency IR photons due to strong and stiffer bond is indicated below,
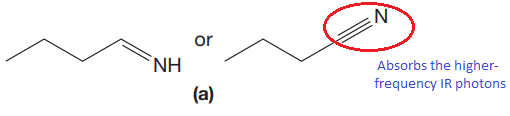
Explanation of Solution
The given pair of compounds is,
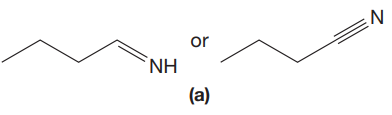
The triple bonds tend to be stronger and stiffer than double bonds. A stronger and stiffer bond tends to lead to a higher vibrational frequency. In given pair of compounds, with the faster vibration, the
The CN stretching mode that absorbs the higher-frequency IR photons is shown below,

The CN stretching mode that absorbs the higher-frequency IR photons is indicated on the basis of the relationship between strength and stiffness of the bond and vibrational frequency.
(b)
Interpretation:
The CN stretching mode that absorbs the higher-frequency IR photons is to be indicated for given pair of compounds. The reason for it is to be explained.
Concept introduction:
We simplify the picture of molecular vibrations by considering the ball-and-spring model, which treats bonds as simple springs that connect atoms together. According to Hooke’s law, the spring vibrates at a particular frequency (
Answer to Problem 15.19P
The CN stretching mode that absorbs the higher-frequency IR photons due to strong and stiffer bond is indicated below,
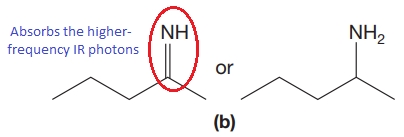
Explanation of Solution
The given pair of compounds is,
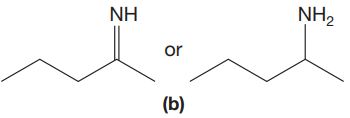
The double bonds tend to be stronger and stiffer than single bonds. A stronger and stiffer bond tends to lead to a higher vibrational frequency. In given pair of compounds, with the faster vibration, the
The CN stretching mode that absorbs the higher-frequency IR photons is shown below,

The CN stretching mode that absorbs the higher-frequency IR photons is indicated on the basis of the relationship between strength and stiffness of the bond and vibrational frequency.
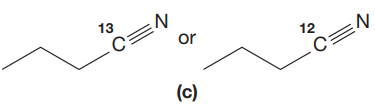
(c)
Interpretation:
The CN stretching mode that absorbs the higher-frequency IR photons is to be indicated for given pair of compounds. The reason for it is to be explained.
Concept introduction:
We simplify the picture of molecular vibrations by considering the ball-and-spring model, which treats bonds as simple springs that connect atoms together. According to Hooke’s law, the spring vibrates at a particular frequency (
Answer to Problem 15.19P
The CN stretching mode that absorbs the higher-frequency IR photons due to lower mass is indicated below,
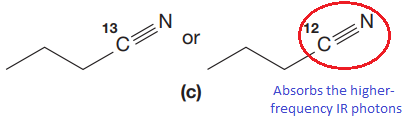
Explanation of Solution
The given pair of compounds is,
In both compounds there is
But
The CN stretching mode that absorbs the higher-frequency IR photons is shown below,

The vibrational mode that absorbs at a higher frequency in the IR region is determined on the basis of the relationship between mass and vibrational frequency.
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY PRINCIPLES & MECHANISM
- Label below statement as True or False. A nucleus that is strongly deshielded requires a lower field strength for resonance.arrow_forwardCopy the defined molecule structure and indicate the configurationabsolute value of each chiral carbon.Attention, the indication will only be considered.if written in front of the carbon to whichshe refersarrow_forwardGive handwritten answer of both subparts.arrow_forward
- answerarrow_forwardGive detailed Solution with explanation needed with structures..don't give Handwritten answerarrow_forwardIn this activity, you will begin to use IR spectroscopy to examine functional groups in organic molecules. First, thinking about what an IR spectrum looks like... An IR spectrum is a most often a plot of... ✓ Choose... wavenumver vs absorbance absorbance vs wavelength transmittance vs wavenumber signal amplitude vs ppmarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,



