
Concept explainers
Interpretation:
Number of moles of each ion per litre in aCaCl2 solution has to be calculated.
Concept Introduction:
Composition of a solution can be defined by expressing its concentration. The concentrations of solutions can be expressed in different ways, which are involved in the quantity of solute and the quantity of solution or solvent. Various methods are used to describe the concentration of the solution quantitatively. Some commonly used quantitative concentration terms are percent by mass, percent by volume, molarity, molality and mole fraction.
Molarity: Molarity is defined as the number of moles of solute present in one litre of the solution In expression,
Again
Answer to Problem 19A
0.221 moles of
Explanation of Solution
Data given: Molarity of
1 mole of
gives 1 mole of 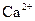 ion and 2 moles of
ion and 2 moles of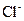 ions. So, 0.221 mole of
ions. So, 0.221 mole of 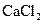 0.221 mole of
0.221 mole of 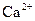 ions and 0.442 moles of
ions and 0.442 moles of 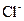 ions, 0.221 mole of
ions, 0.221 mole of 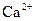 ions and 0.442 mole of
ions and 0.442 mole of 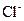 ions are present per litre in a CaCl2 solution.
ions are present per litre in a CaCl2 solution.
Chapter 15 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





