
(a)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a
Answer to Problem 26E
The stoichiometry concept map is shown below.
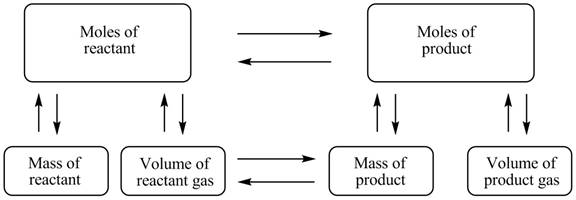
The mass of
Explanation of Solution
It is given that
The given chemical reaction is stated below.
The stoichiometry concept map is shown below.
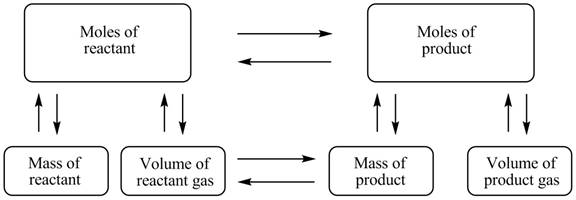
Figure 1
The chemical reaction (1) should be balanced first to calculate the mass of
The number of potassium atom and the hydrogen atom are not equal on both sides. A coefficient of
The balanced equation is stated below.
The equation (2) shows that
At
Therefore, the number of moles of
The molar mass of potassium is
The molar mass of oxygen is
The molar mass of hydrogen is
The molar mass of carbon is
The number of moles of
The mass for
Therefore, the mass of
The stoichiometry concept map is shown in Figure 1. The mass of
(b)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of the
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a chemical reaction. It gives the relationship between the reactants and products. Stoichiometry concept maps are used to determine the relation of reactant and product in terms of stoichiometry.
Answer to Problem 26E
The stoichiometry map is shown below.
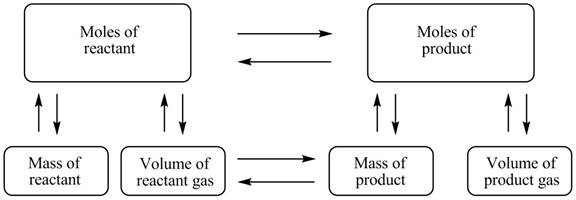
The mass of
Explanation of Solution
The stoichiometry concept mass is shown below.
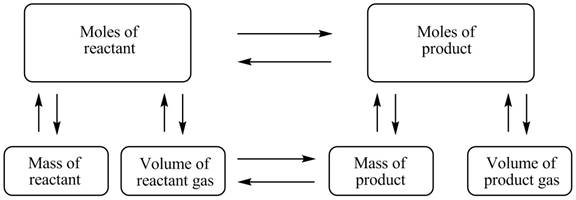
Figure 1
The number of potassium atom and the hydrogen atom are not equal on both sides. A coefficient of
The balanced equation is stated below.
The equation (2) shows that
The molar mass of
The mass calculated of
The number of moles of
The molar mass of potassium is
The molar mass of oxygen is
The molar mass of carbon is
The mass of
The number of moles of
The mass for
Therefore, the mass of
The mass of
Want to see more full solutions like this?
Chapter 15 Solutions
Pearson Etext Introductory Chemistry: Concepts And Critical Thinking -- Access Card (8th Edition)
- Liquid oxygen was first prepared by heating potassium chlorate, KClO3, in a closed vessel to obtain oxygen at high pressure. The oxygen was cooled until it liquefied. 2KClO3(s)2KCl(s)+3O2(g) If 171 g of potassium chlorate reacts in a 2.70-L vessel, which was initially evacuated, what pressure of oxygen will be attained when the temperature is finally cooled to 25C? Use the preceding chemical equation and ignore the volume of solid product.arrow_forward105 The decomposition of mercury(II) thiocyanate produces an odd brown snake-like mass that is so unusual the process was once used in fireworks displays. There are actually several reactions that take place when the solid Hg(SCN)2 is ignited: 2Hg(SCN)2(s)2HgS(s)+CS2(s)+C3N4(s)CS2(s)+3O2(g)CO2(g)+2SO2(g)2C3N4(s)3(CN)2(g)+N2(g)HgS(s)+O2(g)Hg(l)+SO2(g) A 42.4-g sample of Hg(SCN)2 is placed into a 2.4-L vessel at 21°C. The vessel also contains air at a pressure of 758 torr. The container is sealed and the mixture is ignited, causing the reaction sequence above to occur. Once the reaction is complete, the container is cooled back to the original temperature of 21°C. (a) Without doing numerical calculations, predict whether the final pressure in the vessel will be greater than, less than, or equal to the initial pressure. Explain your answer. (b) Calculate the final pressure and compare your result with your prediction. (Assume that the mole fraction of O2 in air is 0.21.)arrow_forwardA student prepares phosphorous acid, H3PO3, by reacting solid phosphorus triiodide with water. PI3(s)+3H2O(l)H3PO3(s)+3HI(g) The student needs to obtain 0.250 L of H3PO3(d=1.651g/cm3). The procedure calls for a 45.0% excess of water and a yield of 75.0%. How much phosphorus triiodide should be weighed out? What volume of water (d=1.651g/cm3) should be used?arrow_forward
- Chloroform, CHCl3, is a volatile (easily vaporized) liquid solvent. Calculate the density of chloroform vapor at 98C and 797 mmHg. Give the answer in grams per liter.arrow_forwardperform stoichiometric ca1cu1uions for reactions involving gases as reactants or products.arrow_forwardThe compression ratio in an automobile engine is the ratio of the gas pressure at the end of the compression stroke to the pressure in the beginning. Assume that compression occurs at constant temperature. The total volume of the cylinder in an automobile is 350 cm3, and the displacement the reduction in volume during the compression stroke is 309 cm3. What is the compression ratio in that engine?arrow_forward
- Ammonium nitrate can be used as an effective explosive because it decomposes into a large number of gaseous products. At a sufficiently high temperature, ammonium nitrate decomposes into nitrogen, oxygen, and steam. (a) Write a balanced net ionic equation for the decomposition of ammonium nitrate. (b) If 2.00 kg of ammonium nitrate are sealed in a 50.0-L steel drum and heated to 745C, what is the resulting pressure in the drum after decomposition? (Assume 100% decomposition.)arrow_forwardButane, C4H10, is an easily liquefied gaseous fuel. Calculate the density of butane gas at 0.857 atm and 22C. Give the answer in grams per literarrow_forwardCarbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: CO(g)+2H2(g)→CH3OH(g) A 2.00 L reaction vessel, initially at 305 K, contains carbon monoxide gas at a partial pressure of 232 mmHg and hydrogen gas at a partial pressure of 386 mmHg. Identify the limiting reactant and determine the theoretical yield of methanol in grams. Express your answer with the appropriate units.arrow_forward
- Since gas stoichiometry always begins with a balanced chemical equation, determine the coefficients necessary to balance the following: _______ C4H10 + 13O2 → _________ CO2 + 10HOH in the presence of heat.arrow_forward7.Since gas stoichiometry always begins with a balanced chemical equation, determine the coefficients necessary to balance the following: ______C4H10 + 13O2 →______CO2 + 10HOH in the presence of heat. Blank 1: _____ Blank 2: _____arrow_forwardIf a bag of fertilizer were labeled as containing 35% K2O, a. What is the analysis when expressed as %K? b. Assume the bag is labeled as 150% P, calculate the percentage P2O5 in the bag.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





