
Concept explainers
(a)
Interpretation:
The concept map is to be drawn and the volume of sulfur trioxide
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is shown below.
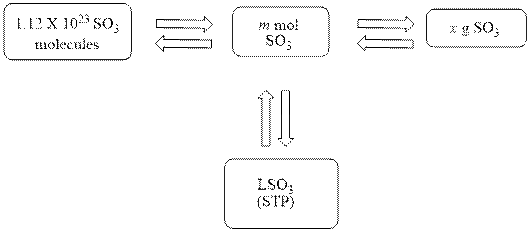
The liters of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The Figure 1 shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The volume of
The number of moles of
The volume for
The volume of
The mole concept map is shown Figure 1. The volume of
(b)
Interpretation:
The mole concept map is to be drawn and the mass of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is drawn below and the grams of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is drawn below.

Figure 1
The above diagram shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The molar mass of oxygen is
The molar mass of sulfur is
The mass of
The number of moles of
The mass for
Therefore, the mass of
The mass of
(c)
Interpretation:
The mole concept map is to be drawn and the molar concentration of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is drawn below and the molar concentration of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The above diagram shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The number of moles for of
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles as
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
Pearson Etext Introductory Chemistry: Concepts And Critical Thinking -- Access Card (8th Edition)
- Write Van der waal’s equation for n moles of a gas.arrow_forwardHow many particles are present in 936 milliliters of hydrogen gas at standard temperature and pressure? to the nearest thousandthsarrow_forwardNitric acid is produced fromnitrogen monoxide, which is in turnis prepared from ammonia by a process known as the Ostwald process, shown below. If 250. mL of oxygen reactswith 250. mL of ammonia, what mass(in g)of nitrogen monoxide will be producedat STP?arrow_forward
- A reaction generates hydrogen gas, H2, as a product. The reactants are mixed and sealed in a 250-mL vessel. After 20.0 minutes, 3.91x10-2 mol H2 have been generated. Calculate the final concentration (in M) of H2 gas.arrow_forwardSuppose instead that the original cylinders contained ½ mol of O2 (g) and 3/2 mol of N2 (g), respectively, each at room temperature and atmospheric pressure. The gases are then mixed at room temperature and allowed to expand to equilibrium against atmospheric pressure. The final volume of the gas mixture.arrow_forward___________ to ____________ 2KClO3 → 2KCl + 3O2 Given Units Unknown Units How many liters of oxygen are produced if 346 grams of KClO3 decomposes @ STP?arrow_forward
- What volume of water is produced when a mixture of 150cm³ of hydrogen and 100cm³ of oxygen is exploded in a eudiometerarrow_forwardIdentify the limiting reactânt when 43.25g of CaC2 reacts with 33.71g of water to form C2H2 and Ca(OH)2arrow_forwardHow many L of water (H2O) are produced if 120.0 moles of oxygen (O2 ) is used?arrow_forward
- State the relationships between the pressure of the gas and the number of gaseous particles.arrow_forward1. calculate the volumetric analysis for Nitrogen in % given the ff Constituent Percentage Oxygen 23.14% Nitrogen 75.53 % Argon 1.28% CO2 0.05%arrow_forwardsolid mercury (II) oxide decomposes when heated forming liquid mercury and oxygen gas as the only products. What mass of mercury (II) oxide must be decomposed in order to collect 843. mL of oxygen gas under STP conditions.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





