
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the liters of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 18E
The concept map is shown below.
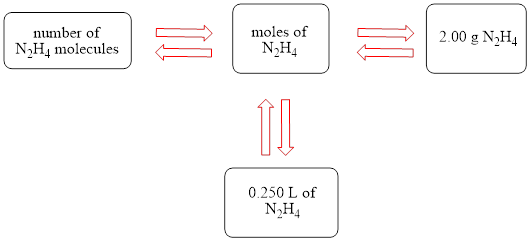
The liters of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The volume occupied by
The formula to calculate the volume occupied by
Substitute the volume of
Therefore, the liters of
The liters of
(b)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 18E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A concept map is to be drawn and molar concentration of the hydrazine solution in the solution in when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 18E
The concept map is shown below.

The molar concentration of the hydrazine solution is
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The number of moles in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
Pearson Etext Introductory Chemistry: Concepts And Critical Thinking -- Access Card (8th Edition)
- How many particles are present in 936 milliliters of hydrogen gas at standard temperature and pressure? to the nearest thousandthsarrow_forwardWhat are the number of moles in one litre of water assume that water density is 1 g/mol.arrow_forwardUse the molar volume to calculate each of the following STP. The number of grams of H2 in 1920 mL of H2 gas express your answer with the appropriate units.arrow_forward
- Draw a diagram representing what is in the container before and after the reaction for a closed container is filled with 2 moles of hydrogen gas and 2 moles of oxygen gas. The gases react to form water.arrow_forwardCauclate number of moles of waterarrow_forwardPotassium hydroxide is used in making liquid soap. How many grams would you use to prepare 2.50L of 1.40M potassium hydroxide?arrow_forward
- if 1.095 g of unknown is found to be 0.00554 moles of solute, what is the molar mass of the unknown.(Your answer should have 3 sig figs)arrow_forwardGrease fires can be extinguished by applying baking soda (sodium hydrogen carbonate), which decomposes due to the heat to produce sodium oxide, carbon dioxide and water. The carbon diocide produced helps to smother the flames. How many grams of baking soda would need to be thrown on to a fire to produce 0.500 moles (approximately 11 L) of carbon dioxide gas?arrow_forwardHousehold bleach contains about 5.2 g of sodium hypochlorite in 100g of water. What is the molarity of sodium hypochlorite in bleach? (Assume no volume change)arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



