
(a)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,
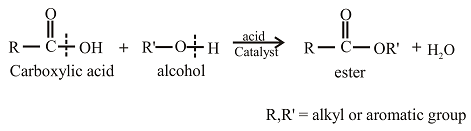
The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,
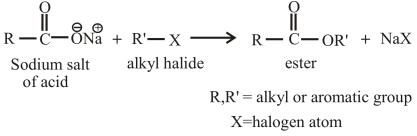
(b)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(c)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(d)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- 1. What are the possible interferences or complications in detecting and differentiating aldehydesand ketones using the following tests:a. 2,4-dinitrophenyl hydrazine test b. benedicts test c. tollens test d. jones test e. iodoform test 2. Describe the positive results for the following qualitative tests:a. 2,4-dinitrophenyl hydrazine testarrow_forwardIdentify the two compounds below that can be prepared from the reaction between a Grignard reagent and an ester.arrow_forwardDescribe the Methods used to Synthesis of Acyl Chlorides or Acyl bromides.arrow_forward
- Include a paragraph to describe the procedure (synthesis of dibenzalacetone) Make sure to discuss the role of the reactants below in the experiment. Sodium hydroxide Ethyl alcohol Acetone Benzaldehydearrow_forwardKhanna et al (1993) found that the following essential oil had a sedative effect more powerful than the drug chlorpromazine (Largactil) and was also an analgesic: Choose one answer. a. black cumin b. neroli c. rose d. ravensaraarrow_forwardIllustrate the Separation of cyclohexanamine and cyclohexanol by an extraction procedure ?arrow_forward
- 4-methoxybenzoic acid is less or more polar than 4-methoxyacetophenone? explain why (WITHOUT DRAWINGS)arrow_forwardShow how you would use anhydrides to synthesize the following compounds. In eachcase, explain why an anhydride might be preferable to an acid chloride.(a) n-octyl formate (b) n-octyl acetate(c) phthalic acid monoamide (d) succinic acid monomethyl esterarrow_forwardWhat happens when an aldehyde gives a silver mirror when treated with Tollen’s reagent and give red-brown precipitate when treated with Fehling’s solution? Explain how they are formed using chemical equations.arrow_forward
- Propose a method to separate a mixture containing phenol, benzoic acid, naphthalene, and p-nitroaniline. Phenol is soluble in sodium hydroxide solution but insoluble in neutral water or sodium bicarbonate solution. Benzoic acid is soluble in either sodium hydroxide or sodium bicarbonate solutions. Write out the structures of the molecules in your scheme.arrow_forwardThe reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, doesnot work with primary alcohols. a. Why does the Ritter reaction not work with primary alcohols? b. Provide an explanation for why an amide is less susceptible to nucleophilic attack than its corresponding ester.arrow_forwardWhy is it not advisable to use aqueous hydrochloric acid in a Grignard reaction of a ketone? A) The Grignard reagent will react with the acid and cannot react with the ketone. B) The ketone will be protonated and will become unreactive. C) The ketone will form an unreactive enol. D) The Grignard reagent won't dissolve in aqueous solutionsarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
