
(a)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
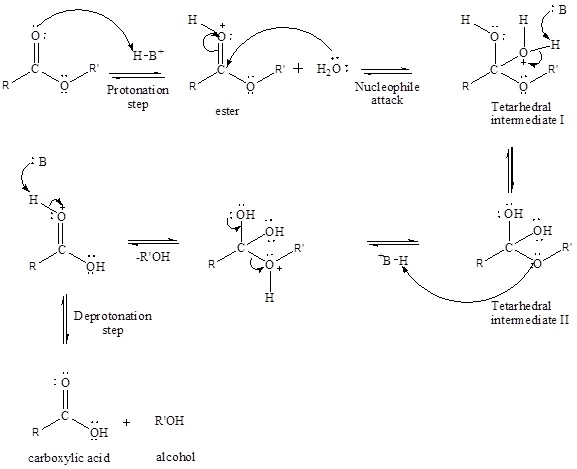
Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(b)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction: An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(c)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- Show the structures of the missing substance(s) in each of the following acid-base equilibria. a. CH3CH2NH2 + H2O ? + OH b. Diethylamine + H2Oarrow_forwardWhat would be the products of the hydrolysis of the following ester?arrow_forwardCardiolipins are found in heart muscles. Draw the products formed when a cardiolipin undergoes complete acid-catalyzed hydrolysis.arrow_forward
- Which product is formed as a result of the decarboxylation of the following molecule?arrow_forwardPlease give the reactions of the following of its esterification reaction: 1. benzoic acid 2. acetic acid + benzyl alcoholarrow_forwardDescribe the Concept of Acid-Catalyzed Ester Hydrolysis and Transesterification.arrow_forward
- Predict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in waterarrow_forwardPropose a reaction for the formation of the following products involving ester formation.arrow_forwardThe final products for the hydroxide-ion-promoted hydrolysis of an ester re the carboxylate ion and methanol instead of the carboxylic acid and methoxide ion. Why?arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



