
Concept explainers
(a) To Determine:
What happens when the lid of a bottle containing chlorine gas is removed?
Answer to Problem 7Q
Solution:
The chlorine gas will mix with room air.
Explanation of Solution
When the lid of the bottle containing the chlorine gas is removed, gas mixes with the air of the room surrounding the bottle and both the bottle and the room is a mixture of chlorine gas and air.
(b) To Determine:
Can reverse process ever happen?
Answer to Problem 7Q
Solution:
No, the reverse process can never happen.
Explanation of Solution
The reverse process that is the chlorine particle recollected so that they settle down in the bottle can never happen because it violates the second law of
(c) To Determine:
Two examples of irreversible process.
Answer to Problem 7Q
Solution:
Irreversible processes are those in which reverse process can never happen. Examples for the same have been given below.
Explanation of Solution
Certain things only happen in one direction and not in the other direction which is called the “arrow of time” and in thermodynamics it is called as entropy which is denoted as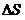 Entropy always increases.
Entropy always increases.
When a food color is added to the glass of water, the color will disperse in some time, but it will never gather again to form a drop of color naturally.So this is an example of irreversible process.
The other example of irreversible process can be viewed as with the passage of time and years, we get older and older but never younger.
Chapter 15 Solutions
Physics: Principles with Applications
Additional Science Textbook Solutions
Conceptual Integrated Science
Modern Physics
Essential University Physics (3rd Edition)
Cosmic Perspective Fundamentals
Tutorials in Introductory Physics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





