
Concept explainers
(a)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In a
Answer to Problem 16.20P
The number of signals with their splitting patterns in the given molecule is indicated as
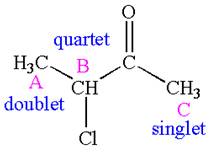
Explanation of Solution
The structure of the given compound is
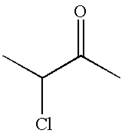
The compound has three chemically distinct protons indicated as
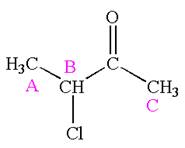
The protons
Splitting of each type of proton is shown below:
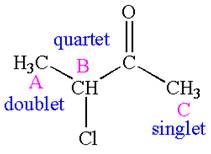
The splitting pattern for each type of proton signal in the given molecule is determined based on their respective number of neighboring protons.
(b)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In a
Answer to Problem 16.20P
The number of signals with their splitting patterns in the given molecule is indicated as
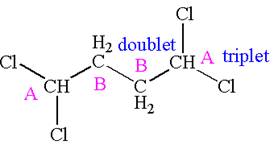
Explanation of Solution
The structure of the given compound is
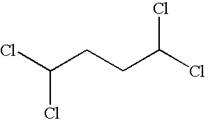
The compound has two chemically distinct protons indicated as
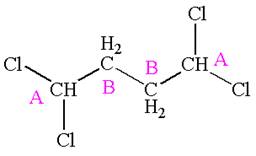
Protons
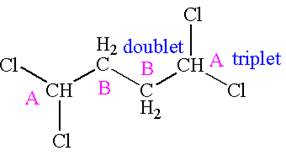
The splitting pattern for each type of proton signal in the given molecule is determined based on their respective number of neighboring protons.
(c)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In a
Answer to Problem 16.20P
The number of signals with their splitting patterns in the given molecule is indicated as
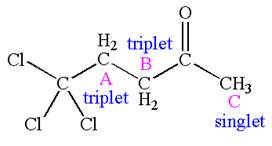
Explanation of Solution
The structure of the given compound is
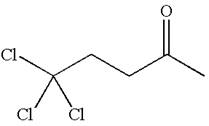
The compound has three chemically distinct protons indicated as
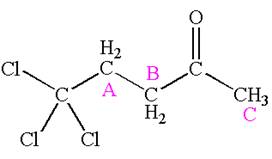
Protons

The splitting pattern for each type of proton signal in the given molecule is determined based on their respective number of neighboring protons.
(d)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In a
Answer to Problem 16.20P
The number of signals with their splitting patterns in the given molecule is indicated as
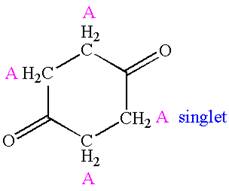
Explanation of Solution
The structure of the given compound is
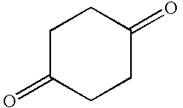
The compound has only one type of protons indicated, so there must be only one signal in
As all protons are chemically equivalent, they do not couple with each other’s signal and appear as singlet.

The splitting pattern for proton signal in the given molecule is determined based on their respective number of neighboring protons.
Want to see more full solutions like this?
Chapter 16 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Which of the highlighted carbon atoms in each molecule absorbs farther downfield?arrow_forwardHow many peaks can you identify from the NMR spectrum? To which does each chemical shift peak corresponds?arrow_forwardInterpret as many of the main peaks in the following spectra as you can... .... .... ...arrow_forward
- Which of the highlighted carbon atoms in each attached molecule absorbs farther downfield ?arrow_forwardMatch the compound to spectrum by drawing the structure next to correct spectrum. Then explain by describing the total number of signals each structure should have and which carbon would be farthest downfield.arrow_forwardWhen looking at your resulting spectrum, the point of maximum absorbance on a particular peak is known as the ____________?arrow_forward
- Draw the correct molecule in the box to the right. Label the relevant peaks in the spectra with the proper functional group. If the absence of key peaks led to your choice, indicate which key peaks were absent.arrow_forwardBased on the spectra you located, does your molecule have a carbonyl? If so, what functional group is it a part of (carboxylic acid, ketone, aldehyde, ester, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a carbonyl? Does your molecule have either an –O-H or –N-H bond? If so, what functional group is it a part of (carboxylic acid, alcohol, amine, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what are the approximate frequency ranges for an –O-H and an –N-H bond? Does your molecule have either an alkyne or nitrile functional group? If so, which functional group is it and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a triple bond?arrow_forwardInto how many peaks will the signal for attached proton be split ?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

