
Concept explainers
Interpretation:
The structure for the compound having molecular formula
Concept introduction:
In
In addition to chemical shift, a
Complicated splitting patterns can result when a proton is unequally coupled to two or more protons that are different from one another.
The ideal range for alkane protons is
Answer to Problem 16.31P
The proposed structure for the compound having molecular formula
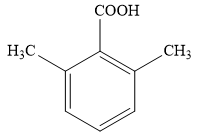
Explanation of Solution
The molecular formula for the given unknown compound is
The molecular formula shows an index of hydrogen deficiency of five.
The
IHD of five and the doublet at
The broad singlet at
The singlet at
For the aromatic protons, the signal at
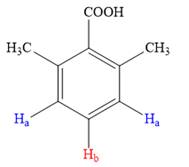
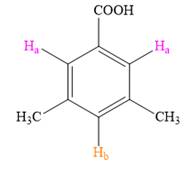
Both the trisubstituted benzenes have only two types of aromatic protons,
In the structure on left, the protons highlighted in blue will appear as a doublet as both of them have identical chemical environment, while the proton in red will split into a triplet as it has two neighboring protons attached.
In the structure on right, the protons in pink will appear as singlets, as they do not have any neighboring protons while the proton in orange will also appear as a singlet.
Thus, the second structure doesn’t match with the data given in the
The
The
The signal
Considering both the
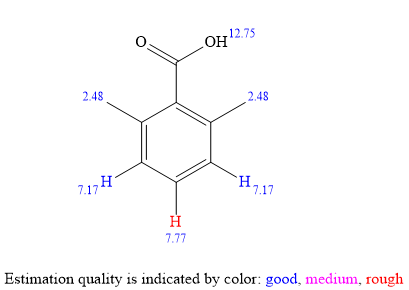
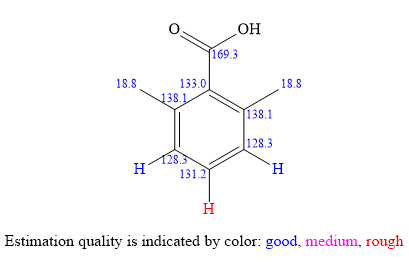
The structure of the unknown compound can be proposed based on its
Want to see more full solutions like this?
Chapter 16 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- V.) A compound with molecular formula C8H10O has the following proton NMR spectrum. Determine the number of protons giving rise to each signal (a, b, c, and d). (SEE PICTURE ATTACHED).arrow_forwardBased on the spectra you located, does your molecule have a carbonyl? If so, what functional group is it a part of (carboxylic acid, ketone, aldehyde, ester, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a carbonyl? Does your molecule have either an –O-H or –N-H bond? If so, what functional group is it a part of (carboxylic acid, alcohol, amine, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what are the approximate frequency ranges for an –O-H and an –N-H bond? Does your molecule have either an alkyne or nitrile functional group? If so, which functional group is it and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a triple bond?arrow_forwardHow many unique signals in HNMR will this compound have? Can you draw them out so I can see?arrow_forward
- Which compound gives a signal in the 1H-NMR spectrum with a larger chemical shift, furan or cyclopentadiene? Explain.arrow_forwardCan you help me determine the structure of the compound represented in this spectrum?arrow_forwardNeed help to describe the proton NMR spectra to distinguish between pair.arrow_forward
- Each line in a 13CNMR spectrum corresponds to a different kind of carbon atom. How many 13CNMR signals does each compound exhibit? ( please explain)arrow_forwardCan you please confirm if this H-NMR spectrum belongs to this molecule and identify the signals of each spectrum.arrow_forwardPlease help me to interpret this spectrum.arrow_forward
- colorcode/ or number ALL of the homotopic protons of the structure. Then choose which group would have the highest shift value, and which group of H would have the lowest shift valuesarrow_forwardHow do I interpret, label, and determine which molecule this H NMR graph represents? The three molecule choices are methylene cyclohexane, 3-methylcyclohexene, or 1-methylcyclohexene.arrow_forwardHow many different signals would you see in the carbon NMR of the compound below? Assume you can see them all.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
