
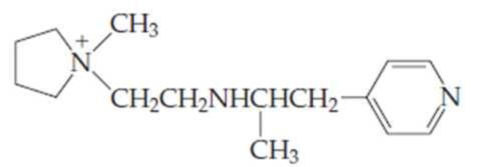
- (a) For the compound above, identify each nitrogen as either a primary, secondary, tertiary, quaternary, or aromatic
amine . - (b) Which amine group(s) would be able to provide a hydrogen bond? Which could accept a hydrogen bond?
(a)
Interpretation:
It should be identified that the each nitrogen in the given compound either as a primary, secondary, tertiary amine, or aromatic amine.
Concept introduction:
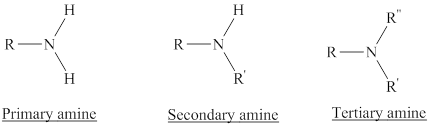
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Example: Tetramethylammonium ion
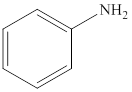
Aniline is an aromatic amine compound and its structure is,
Answer to Problem 16.23UKC
Explanation of Solution
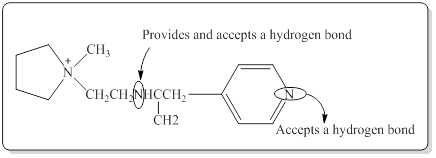
Structure of the given compound is,
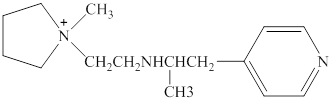
As per the concepts above mentioned,
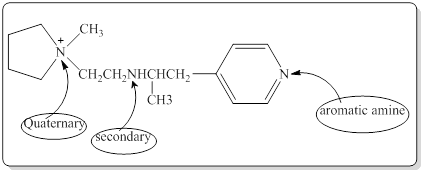
Each nitrogen atoms in the given compound can be identified either as a primary, secondary, tertiary amine or aromatic amine as follows,
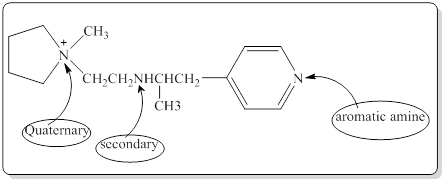
(b)
Interpretation:
The amine group which would be able to provide a hydrogen bond and accept a hydrogen bond in the given compound has to be identified.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Hydrogen bond is an attractive force established between hydrogen atom attached to a highly electronegative element and another highly electronegative element of the same or different molecule.
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
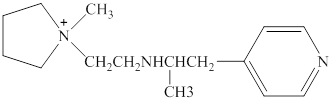
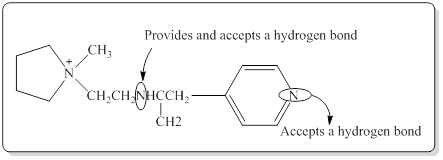
In a hydrogen bond strong partial positive charge on hydrogen attracts lone electron pair on oxygen or nitrogen.
Here in the given molecule, an amine group which was able to provide a hydrogen bond and accept a hydrogen bond and those amines can be identified as follows,
Want to see more full solutions like this?
Chapter 16 Solutions
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
- a) Use the carbonyl group, number of carbons, and type of stereochemistry (D or L) to classify the following monosaccharides. CH2OH O: CHO I но -H- HO- но -H- HOCH2 HO- -O- CH2OH H- OH CH2OH i.Compound A is a(n with stereochemistry. ii.Compound B is a(n) with stereochemistry.arrow_forwardDraw the complete structural formula of arachidonic acid (Table 23.1) in a way that shows the cis stereochemistry of its four double bonds.arrow_forwardFor the following molecule, I need to identify several things, but I don't understand how to go about it. a) anomeric carbon b) carbon 1 c) carbon 5 d) which oxygen atoms in a hydroxyl group would point to the right in a Fischer Projectionarrow_forward
- A) Describe the glycosidic bond (using standard convention) indicated by “Arrow a.” B) Draw the open chain Fischer projection formula of the monosaccharide labeled “B” C) Describe the glycosidic bond (as in question A) indicated by “Arrow b.”arrow_forwardIdentify the chiral carbon in each of the following compounds: a. citronellol; one enantiomer has the odor of geranium b. alanine, an amino acidarrow_forwardIDENTIFY THE FUNCTIONAL GROUP PRESENT IN THESE COMPOUNDS. A = ? B = ? C = ?arrow_forward
- Consider each of the following disaccharides: a) Label the acetal and hemiacetal in each disaccharide. b) Number the carbons in each of their monosaccharide rings. c) Classify the glycosidic linkage as α or β and use numbers to designate its location. d) Draw the Fishcher projections of the monosaccharides formed when each of thedisaccharides is hydrolyzed? e) Draw the chair conformation of each monosaccharide, where applicable.arrow_forwardFollowing is a structural formula for a disaccharide. a) Name the two monosaccharide units in the disaccharide. b) Describe the glycosidic bond.arrow_forwardWhich of the structures below DOES NOT correspond to a "correct" (i.e. probable or possible) structure for a monosaccharide? (remember that unlabeled vertices are carbon atoms) a) A b) B c) C d) Darrow_forward
- Why is a thioester bond a “high-energy” bond?arrow_forwardCompound A is a dipeptide, optically inactive. While compound B is a tripeptide, and optically active. Compound A is formed when compound C and compound D joined together by condensation reaction. Whereas monomers E and F are formed by modifying the compounds C and D. Polymer G is formed by the condensation reaction of monomers E and F. Draw the possible structural formulae A, B,C,D,E,F and polymer G. Label the peptide bond(s) for compounds A and B. Pls name the compounds explain tooarrow_forwardCONH, HQ NH (CH)3C a.) Put asterisk on chiral centers b.) label each chiral center as R or S. c.) Draw the enantiomer d.) Draw one diastereomerarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
