
Concept explainers
(a)
Interpretation:
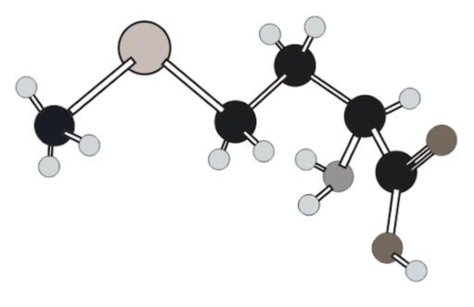
Amino acid in ball and stick model below has to be determined.
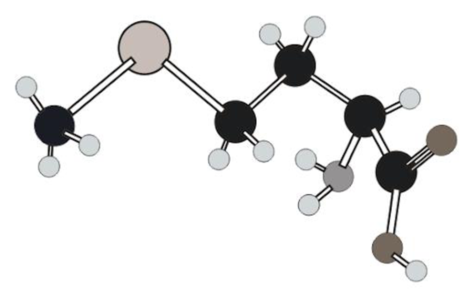
Concept Introduction:
Amino acids are the
(1) Neutral amino acids exist in their zwitterionic form at
(2) Amino acids exist with net
(3) Amino acids exist with net
(4) At physiological
(5) Value of
(6) Isoelectric point of amino acids with additional carboxylic group is around 3.
(7) Isoelectric point of amino acids with additional basic nitrogen atom is around
(a)
Explanation of Solution
In ball and stick model different atoms are as follows:
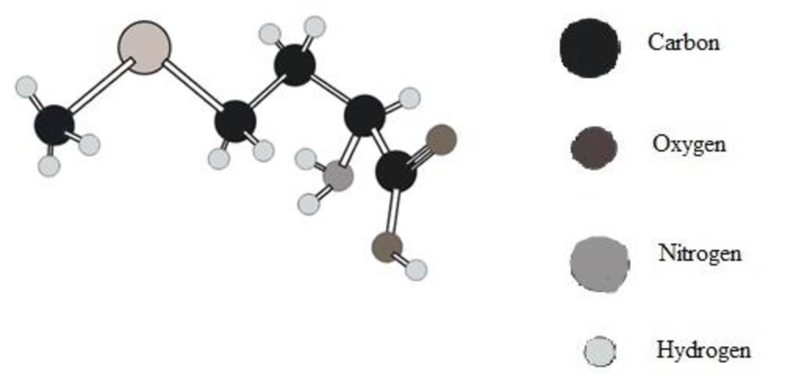
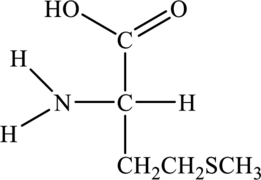
Amino acid can be drawn as follows:
(b)
Interpretation:
Three-letter and one-letter abbreviations for amino acid in ball and stick model below have to be determined.
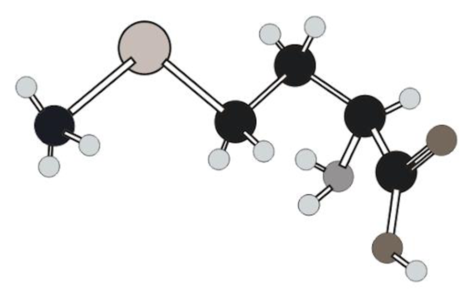
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Structure of given amino acid as follows:
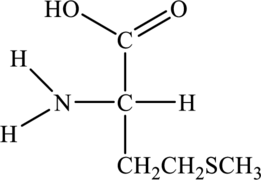
Since amino acid has
(c)
Interpretation:
The form that presents at isoelectric point for amino acid represents below has to be determined.
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
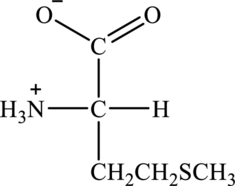
Structure of given amino acid as follows:
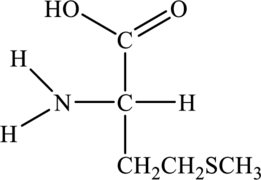
Since amino acid has
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, & Biological Chemistry
- Identify the R group of the side chain in the following amino acids that results in the side-chain classification indicated in parentheses see Table 19.1: a. tyrosine neutral, polar b. glutamate acidic, polar c. methionine neutral, nonpolar d. histidine basic, polar e. cysteine neutral, polar f. valine neutral, nonpolararrow_forwardWhich amino acids could be referred to as derivatives of butanoic acid?arrow_forwardWhich amino acid has the lowest pI value?arrow_forward
- Name each peptide using both the three-letter and one-letter abbreviations of the component amino acids.arrow_forwardIdentify the R group of the side chain in the following amino acids that results in the side-chain classification indicated in parentheses a. cysteine (neutral, polar)b. arginine (basic, polar)c. valine (neutral, nonpolar)d. aspartate (acidic, polar)arrow_forwardHow to identify this amino acid?arrow_forward
- (A) How many peptide bonds are present in peptide 1? (B) What is the N-terminal amino acid in peptide 2? (C) What is the C-terminal amino acid in peptide 2?arrow_forwardidentify the amino acid described. state the name, one-letter abbreviation, and draw the structure at pH 12.0 a polar amino acid with 2 chiral centersarrow_forwardIdentify one amino acid from each of the following categories:arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



