
Concept explainers
(a)
Interpretation:
The product formed in the reaction of
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to
Answer to Problem 16.51P
Explanation of Solution
Aldehydes when made to react with potassium dichromate are known to form the corresponding carboxylic acids. The reaction of hexanal in the presence of potassium dichromate will result in the formation of hexanoic acid as shown below in the chemical equation:
Hence the reaction of hexanal with potassium dichromate results in the formation of the corresponding carboxylic acid which is hexanoic acid.
(b)
Interpretation:
The product formed in the reaction of

Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 16.51P
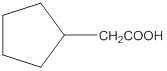
Explanation of Solution
Aldehydes when made to react with potassium dichromate are known to form the corresponding carboxylic acids. The reaction of 2-cyclopentylacetaldehyde in the presence of potassium dichromate will result in the formation of 2-cyclopentanoic acid as shown below in the chemical equation:
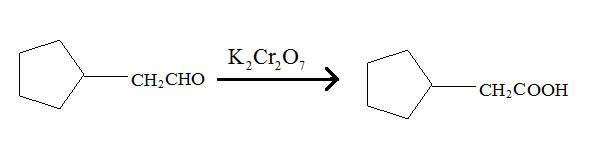
Hence the reaction of 2-cyclopentylacetaldehyde with potassium dichromate results in the formation of the corresponding carboxylic acid which is 2-cyclopentylacetic acid.
(c)
Interpretation:
The product formed in the reaction of
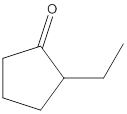
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 16.51P
No product formed.
Explanation of Solution
The given compound is 2-ethylcyclopentanone and it cannot react with potassium dichromate as it contains ketone as a functional group and potassium dichromate does not oxidize
(d)
Interpretation:
The product formed in the reaction of
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 16.51P
Explanation of Solution
The given structure clearly shows that this compound is hexanol. It is an aliphatic alcohol and it will react with potassium reagent to form the corresponding aldehyde which will further get oxidized and the reaction will finally result in the formation of a carboxylic acid.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- 3. a. What is the chemical structure of benzoic acid, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forwarda. What is the chemical structure of 2,6-dichloroindophenol, circle functional groups differentthan alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? ___________arrow_forwarda. What is the chemical structure of biphenyl, circle functional groupsdifferent than alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward
- This carboxylic salts are effective ingredient against yeast and molds in beverages, jams, pie fillings and ketchup a. benzoates b. sorbates c. acetates d. propionatesarrow_forwarda. What is the chemical structure of biphenyl? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forwardWhat is the product using these reagents? 1) Mg, Et2O; 2) D2O.arrow_forward
- Draw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 14O b. an ether with molecular formula C 6H 14O that has a methyl group bonded to oxygen c. a 3 ° alkyl halide with molecular formula C 5H 11Brarrow_forwardWhat product is formed when benzene is treated with each organic halide in the presence of AlCl3?arrow_forwardList the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forward
- 1. what grouo does the ff organic compound belong? a. ketone b. ether c. cyloalkane d. esther 2. what group does the ff organic compound belong? a. amide b. azo c. nitrile d. amine 3. what is the priority functional group of the ff organic compound? a. carboxyl b. hydroxyl c. carbonyl d. hydroxidearrow_forwardDraw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 12O b. a cyclic ether with molecular formula C 5H 10O c. a 1 ° alkyl halide with molecular formula C 5H 11Clarrow_forwardDraw the product of benzene and Cl2/FeCl3arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


