
(a)
Interpretation:
The appropriate number with the quantity reactants needs to be matched.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
Answer to Problem 45A
Location 2 represents thereactants molecules.
Explanation of Solution
In the figure,
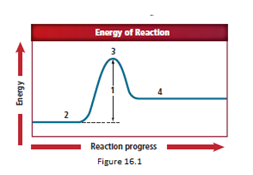
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
In a reaction, reactants are first thing that appear and it have the lowest energy in an exothermic reaction.
So, the correct answer for reactants is location 2.
(b)
Interpretation:
The appropriate number with the quantity activated complex needs to be matched.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(b)
Answer to Problem 45A
Location 3 represents the activated complex.
Explanation of Solution
In the figure,
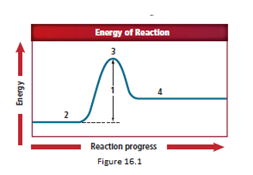
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. After the crossing of the barrier, an activated complex starts forming.
So, as location 3 comes after location 2, the correct answer representing an activated complex is location 3.
(c)
Interpretation:
To match the appropriate number with the quantity products represents.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(c)
Answer to Problem 45A
Location 4 represents the product.
Explanation of Solution
In the figure,
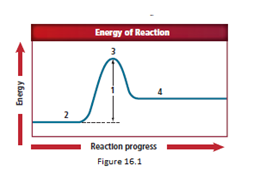
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
After the formation of the complex, the products start forming.
So, as location 4 comes after location 3, the correct answer representing an activated complex is location 4.
(d)
Interpretation:
To match the appropriate number with the quantity activation energyrepresents.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(d)
Answer to Problem 45A
Location 1 represents the activation energy.
Explanation of Solution
In the figure,
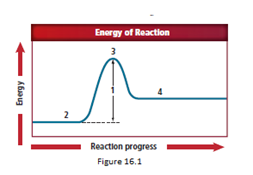
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
The activation energy value is the difference in energy value of reactants and activated complex.
Location 1 represents the activation energy, as the difference in energy is designated by location 1.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Essential Organic Chemistry (3rd Edition)
Chemistry: A Molecular Approach
Organic Chemistry (8th Edition)
CHEMISTRY-TEXT
General Chemistry: Principles and Modern Applications (11th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





