
Use the diagram below to answer Questions 8 and 9.
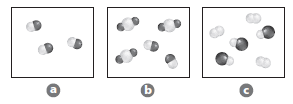
Which sample could contain particles of magnesiumfluoride?
A. a
B. b
C. c
D. Both a and b
Interpretation:
The sample that contain particles a of magnesium fluoride from the given figure needs to be determined.
 Concept introduction:
Concept introduction:
Diatomic molecules consists two atoms of different or same elements. For example,
Answer to Problem 9STP
B.b
Explanation of Solution
B. magnesium fluoride has three atoms - one magnesium and two fluorine atoms. Two fluorine atoms are attached to the magnesium atom. The given figure shows different size and colors. Option B has three atoms which attached to each other with larger central atom and two atoms are the same in color.
The incorrect options are as follows:
A. a
Figure a has different atoms that attached one another.
C. c
Figure c has different atoms and two identical atoms that attached one another.
D. both a and b
Both figure a has different atoms.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Organic Chemistry (9th Edition)
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





