
Concept explainers
(a)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from
Answer to Problem 17.53P
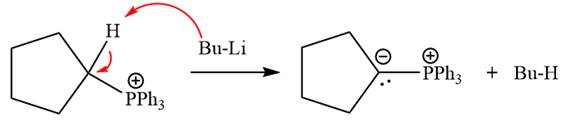
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
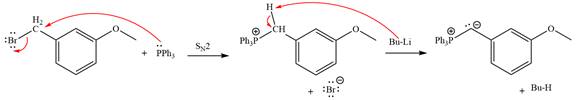
Explanation of Solution
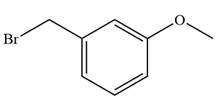
The given alkyl halide is
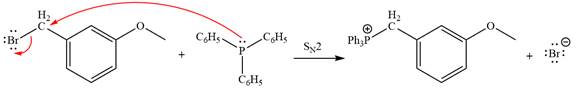
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
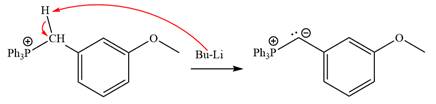
Lastly, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
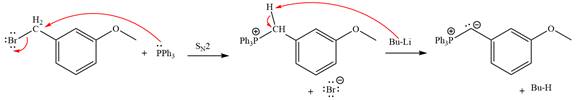
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
(b)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from alkyl halides. The alkyl halide is first reacted with triphenylphosphine and the product of that reaction is treated with a strong base. In the first step reaction fovors
Answer to Problem 17.53P
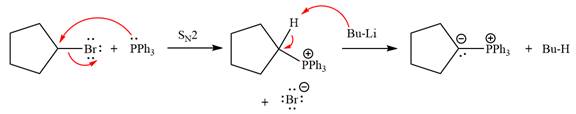
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
Explanation of Solution
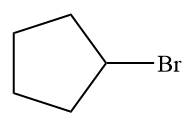
The given alkyl halide is
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
Lastly, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
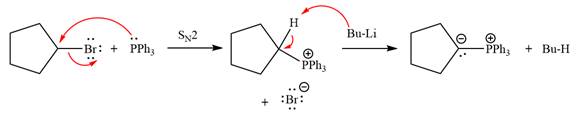
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
(c)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from alkyl halides. The alkyl halide is first reacted with triphenylphosphine and the product of that reaction is treated with a strong base. In the first step reaction fovors
Answer to Problem 17.53P
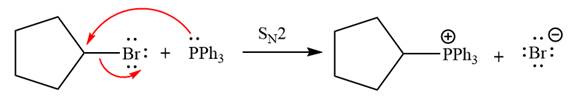
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
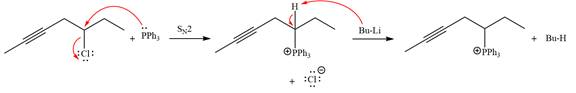
Explanation of Solution
The given alkyl halide is
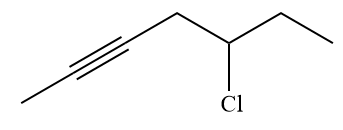
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
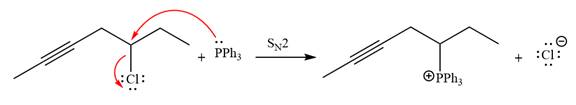
After that, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
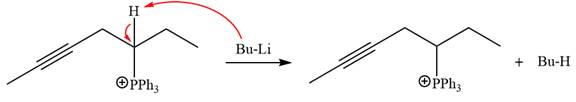
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
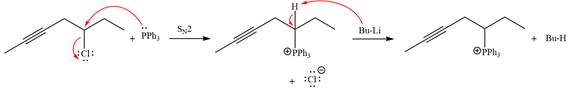
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- The reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forwardDraw a complete, detailed mechanism AND predict the major organic product for the following transformations a, b & c.arrow_forwardDraw a detailed mechanism for the following reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





