
Concept explainers
(a)
Interpretation:
The synthesis of the given
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
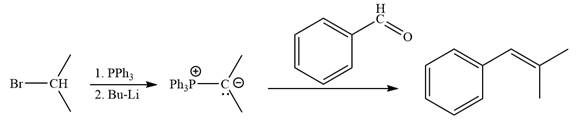
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is shown below:
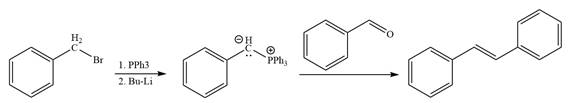
Explanation of Solution
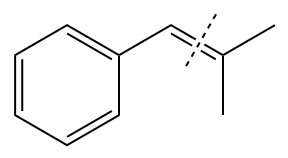
The given structure of alkene is
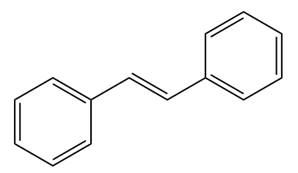
In the above structure, the
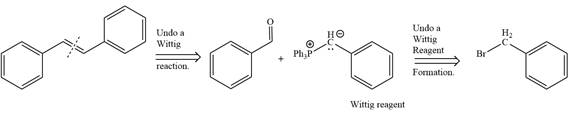
By undoing a Wittig reaction in retrosynthetic analysis,
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehydewould appears as follows:
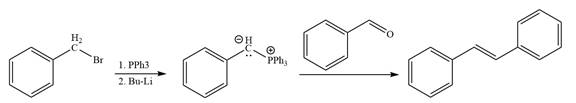
The synthesis of given alkene from an alkyl halide and a ketone or aldehyde is shown.
(b)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
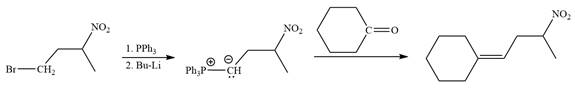
Method 2:
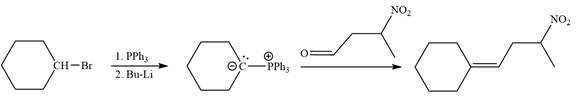
Explanation of Solution
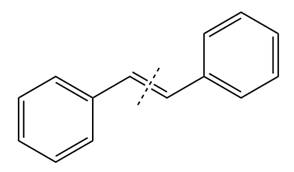
The given structure of alkene is
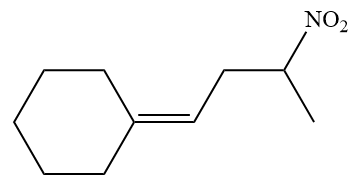
In the above structure, the
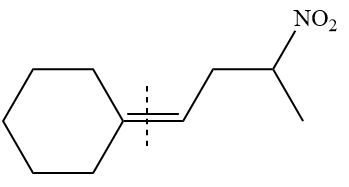
Method 1:
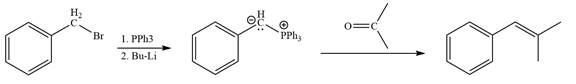
By undoing a Wittig reaction in retrosynthetic analysis,
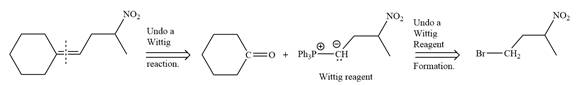
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:
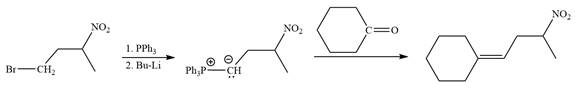
Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
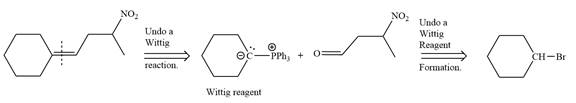
In the forward direction, the synthesis of the given alkene from an alkyl halide and an aldehyde would appear as follows:

The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
(c)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
Method 2:
Explanation of Solution
The given structure of alkene is
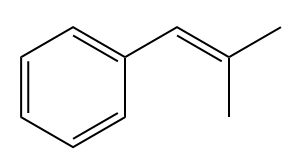
In the above structure, the
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
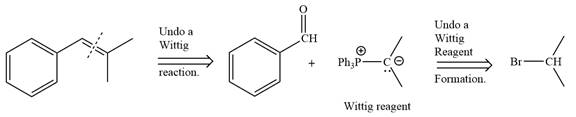
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehyde would appear as follows:

Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
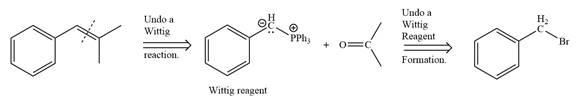
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:

The synthesis of the given unsymmetric alkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
Want to see more full solutions like this?
Chapter 17 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- please draw the complete, detailed mechanism and the product of this reaction.arrow_forward(SYN) Show how to synthesize the following molecule from any compounds containing two carbons. Draw the complete, detailed mechanism for the reaction.arrow_forward(SYN) Show how to carry out each of the following syntheses by first converting the alcohol into a sulfonyl chloride.arrow_forward
- Draw the complete, detailed mechanism for each of the following reactions and predict the major products.arrow_forwardDraw the complete, detailed mechanism and the products for each of the following reactions.arrow_forwardDraw the complete, detailed mechanism for the following reaction.arrow_forward
- Draw a complete, detailed mechanism for this reaction.arrow_forward(SYN) Show how to synthesize each of the following trisubstituted benzenes from a disubstituted benzenearrow_forwardHelp, explain in detail please. Thank you! Did the overal reaction shown here occur by and SN2, Sn1, E2, or E1 mechanism? How do you know? Draw the complete, detailed mechanism to account for the formation of both products.arrow_forward
- Textbook problem: Suggest short series of reactions that would be expected to transform the material on the right into the desired product shown on left.arrow_forwardplease correctly give detail mechanism of formation of both the products. I repeat give mechanism for both the product .arrow_forwardDetermine the major product of each reaction in Problem and draw the complete, detailed mechanism. Pay attention to stereochemistry where appropriate.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





