
(a)
Interpretation:
The complete, detailed mechanism for the given reaction is to be drawn. The major product is to be predicted.
Concept introduction:
Phosphorus tribromide (
Answer to Problem 17.67P
The complete, detailed mechanism and the major product for the given reaction is drawn as shown below:
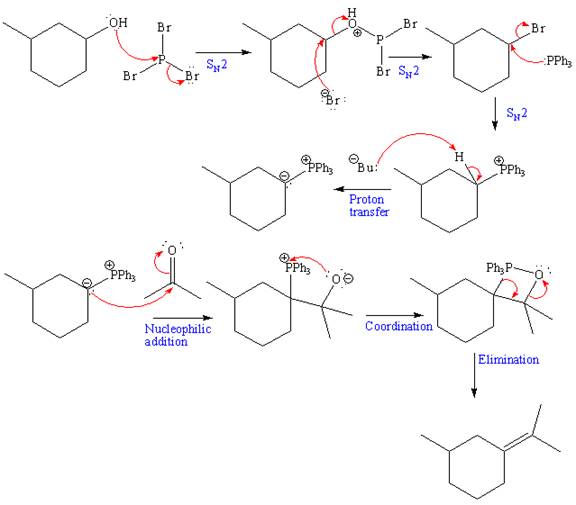
Explanation of Solution
The given starting material and reagents are as shown below:
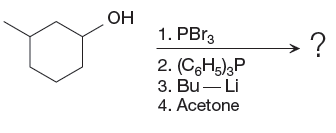
The reagent used for the first step of this reaction is Phosphorus tribromide (
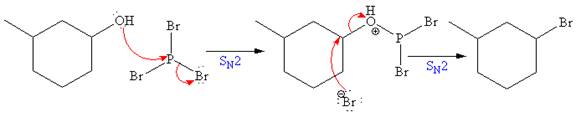
The above formed alkyl bromide further undergoes an
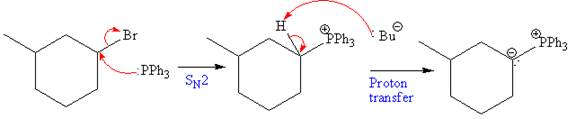
The above Wittig reagent undergoes Wittig reaction with acetone. In a Wittig reaction, the
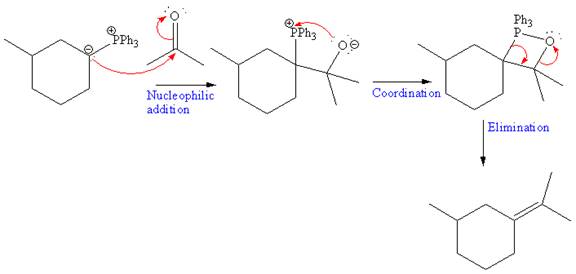
The overall mechanism and the major product of the given reaction is as shown below:
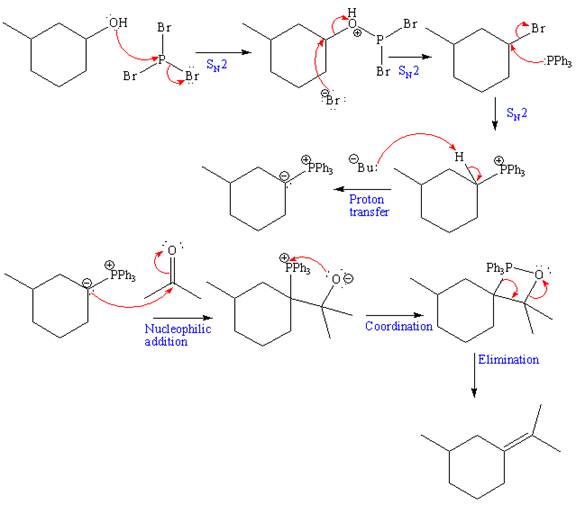
The product of the given reaction and complete mechanism is drawn on the basis of the given reagents.
(b)
Interpretation:
The complete, detailed mechanism for the given reaction is to be drawn. The major product is to be predicted.
Concept introduction:
The
Answer to Problem 17.67P
The complete, detailed mechanism and the major product for the given reaction is drawn as shown below:
Explanation of Solution
The given starting material and reagents are as shown below:
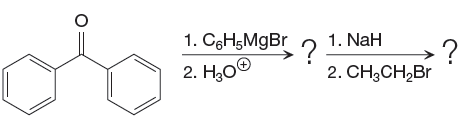
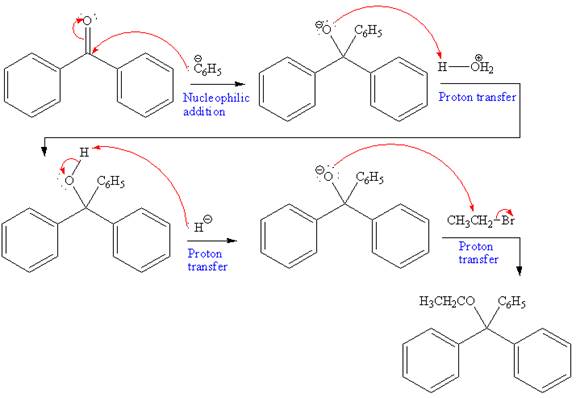
In the first reaction, a new C-C bond is formed and a phenyl group is added to the carbonyl carbon. Grignard reagents can react rapidly with
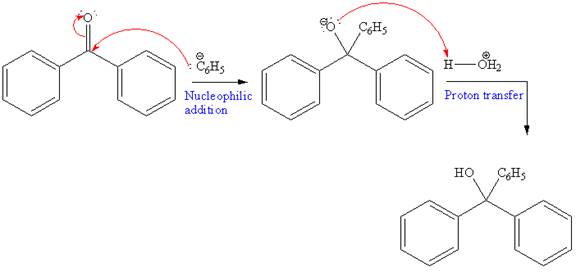
Further, this alcohol undergoes deprotonation by NaH to form negatively charged alkoxide. This alkoxide acts as a strong nucleophile and attacks ethyl bromide via
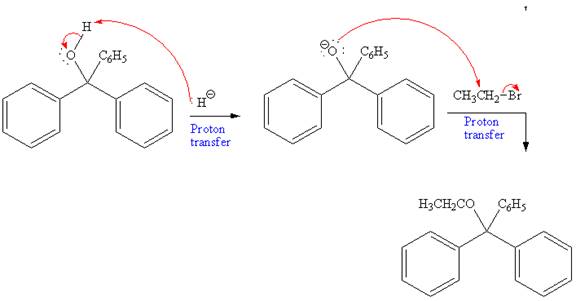
The overall mechanism and the major product of the given reaction are as shown below:
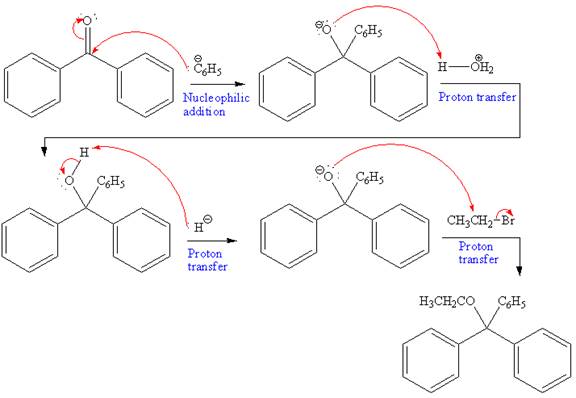
The product of the given reaction and complete mechanism is drawn on the basis of the mechanism for Grignard reagent and Williamson ether synthesis.
Want to see more full solutions like this?
Chapter 17 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Predict the major products of the following reactions. Draw the complete, detailed mechanism that leads to the formation of each of those products.arrow_forwardplease draw the complete, detailed mechanism and the product of this reaction.arrow_forwardDraw the complete, detailed mechanism for the reaction shown here. Will the product be optically active? Explain.arrow_forward
- Draw the complete, detailed mechanism for the following reaction.arrow_forward(SYN) Show how to synthesize the following molecule from any compounds containing two carbons. Draw the complete, detailed mechanism for the reaction.arrow_forwardDraw a complete, detailed mechanism for this reaction.arrow_forward
- Draw the complete mechanism and the major organic product for each of the following reactions.arrow_forwardDraw a complete, detailed mechanism for the following reaction. A key intermediate is provided.arrow_forwardPlease draw the complete, detailed mechanism of the reaction step by step.arrow_forward
- Determine the major product of each reaction in Problem and draw the complete, detailed mechanism. Pay attention to stereochemistry where appropriate.arrow_forwardDraw a complete, detailed mechanism AND predict the major organic product for the following transformations a, b & c.arrow_forwardGive the detailed mechanism of the reaction and form major product!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





