
Concept explainers
(a)
Interpretation:
The ester formed on treating 2-propanol with CH3CH2COOH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
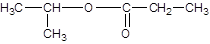
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2COOH (propanoic acid) and 2-propanol is:
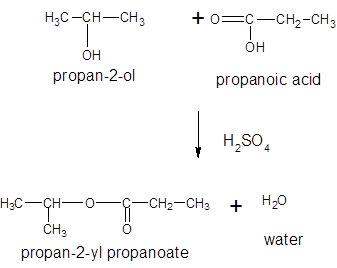
(b)
Interpretation:
The ester formed on treating 2-propanol with following in the presence of H2SO4 should be determined:
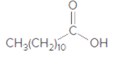
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
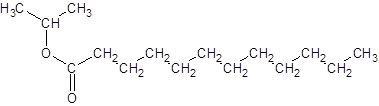
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3(CH2)10COOH (dodoecanoic acid) and 2-propanol is:
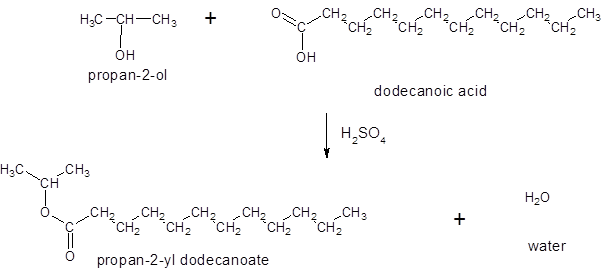
(c)
Interpretation:
The ester formed on treating 2-propanol with HCO2H in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between HCO2H (formic acid) and 2-propanol is:
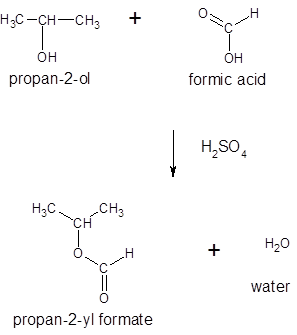
(d)
Interpretation:
The ester formed on treating 2-propanol with following in the presence of H2SO4 should be determined:
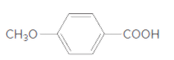
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
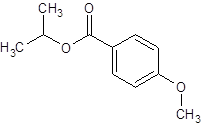
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between 4-methoxybenzoic acid and 2-propanol is:
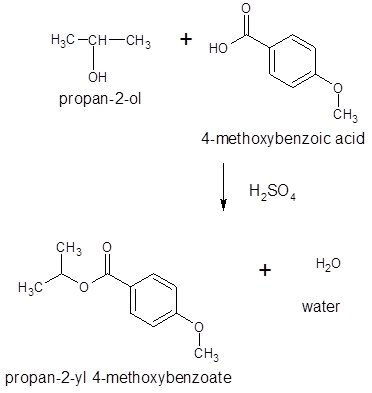
Want to see more full solutions like this?
Chapter 17 Solutions
GENERAL ORGANIC+BIOCHEM (LL)W/CONNECT
- Draw the carbonyl products formed when each alcohol is oxidized with K 2Cr 2O 7.arrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. a. HNO3, H2SO4 h. product in (a), then Sn, HClarrow_forwardList the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forward
- This carboxylic salts are effective ingredient against yeast and molds in beverages, jams, pie fillings and ketchup a. benzoates b. sorbates c. acetates d. propionatesarrow_forward1. What are the characteristics of a positive tollens test for adehydes? What is the oxidizing agent in tollens solutions? 2. What is the characteristics of a positive Benedict's test for aldehydes? What is the oxidizing agent in Benedict's solution?arrow_forwardwhich organic compound dissolves in water? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanol which organic compound dissolves in 10% NaOH? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanolarrow_forward
- Draw the reaction of butane-1,2-diol with (CH3)2COarrow_forward1. What is the role of the acetic acid in the oxidation of Cyclohexanol to Cyclohexanone? Write the balanced chemical reaction between acetic acid and sodium hypochlorite.2. How do you neutralize the acetic acid regenerated in the reaction? Write the balanced chemical reaction.arrow_forwardCompounds that contain both a hydroxyl group (OH) and a carboxyl group (COOH) can undergo an intramolecular esterifi cation reaction. What product is formed when each hydroxy acid undergoes an intramolecular reaction? a. HOCH 2CH 2CH 2CH 2CO 2H b. HOCH 2CH 2CH 2CO 2Harrow_forward
- 1. Carboxylic acid reacts with an alcohol to form: A. Ester and Water B. Ester C. Water D. No reaction 2. The general formula for Carboxylic acids: A. RCOOH B. RCOOR C. RCOR D. RCOH 3. General formula of phenols: * A. ROH B. Ar-OH C. R-SH D. RCOHarrow_forwardDraw the product formed when the alcohol cyclobutanol is dehydrated with H2SO4.arrow_forwardDraw the products formed when each alcohol is dehydrated with H 2SO 4. Use the Zaitsev rule to predict the major product when a mixture forms.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


