
(a)
Interpretation:
The given reaction is
The reaction quotient,
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
(a)
Explanation of Solution
The given reaction is
The reaction quotient for the reaction can be represented as
(b)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
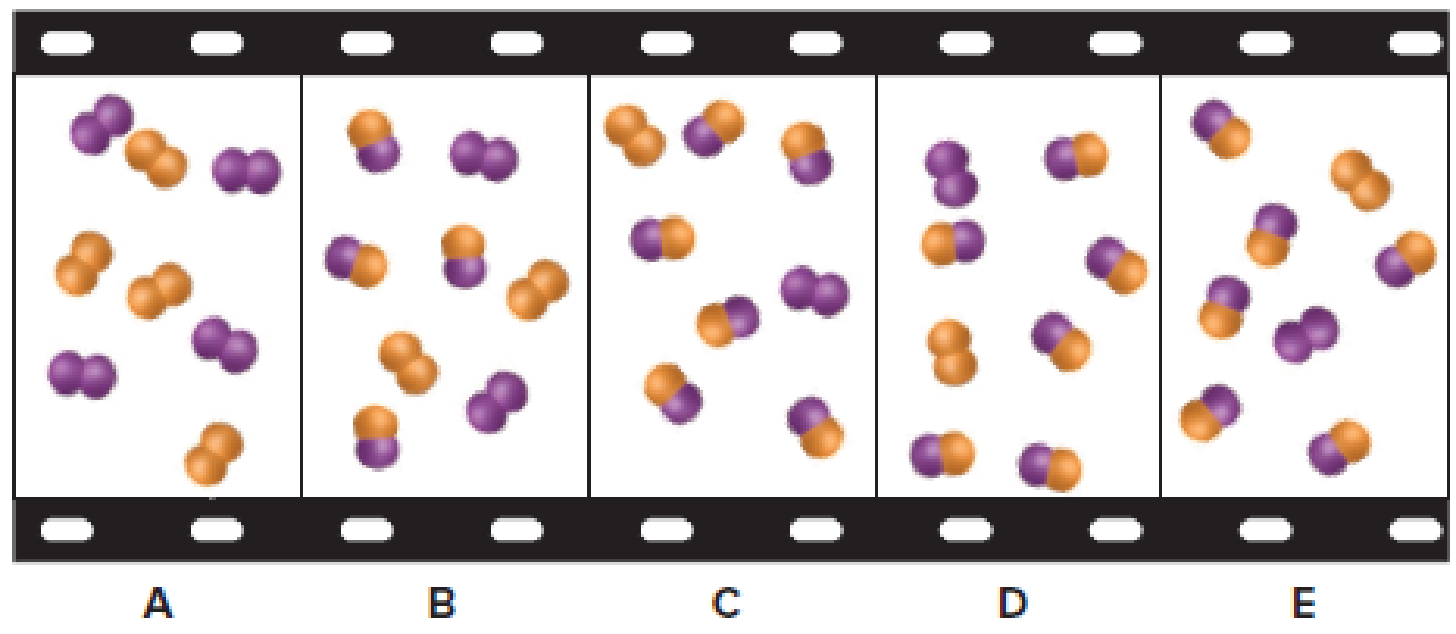
Figure 1
If each particle represents
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
(b)
Explanation of Solution
Scene A:
The
Scene B:
The
Scene C, D, E:
The
(c)
Interpretation:
The given reaction is
If
Concept Introduction:
At equilibrium,
If
If
If
(c)
Explanation of Solution
The given condition is
Scene-A:
The value of
Scene-B:
The value of
Scene-C, D, E:
The value of
As the value of
(d)
Interpretation:
The given reaction is
The value of
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
At equilibrium,
(d)
Explanation of Solution
As
(e)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
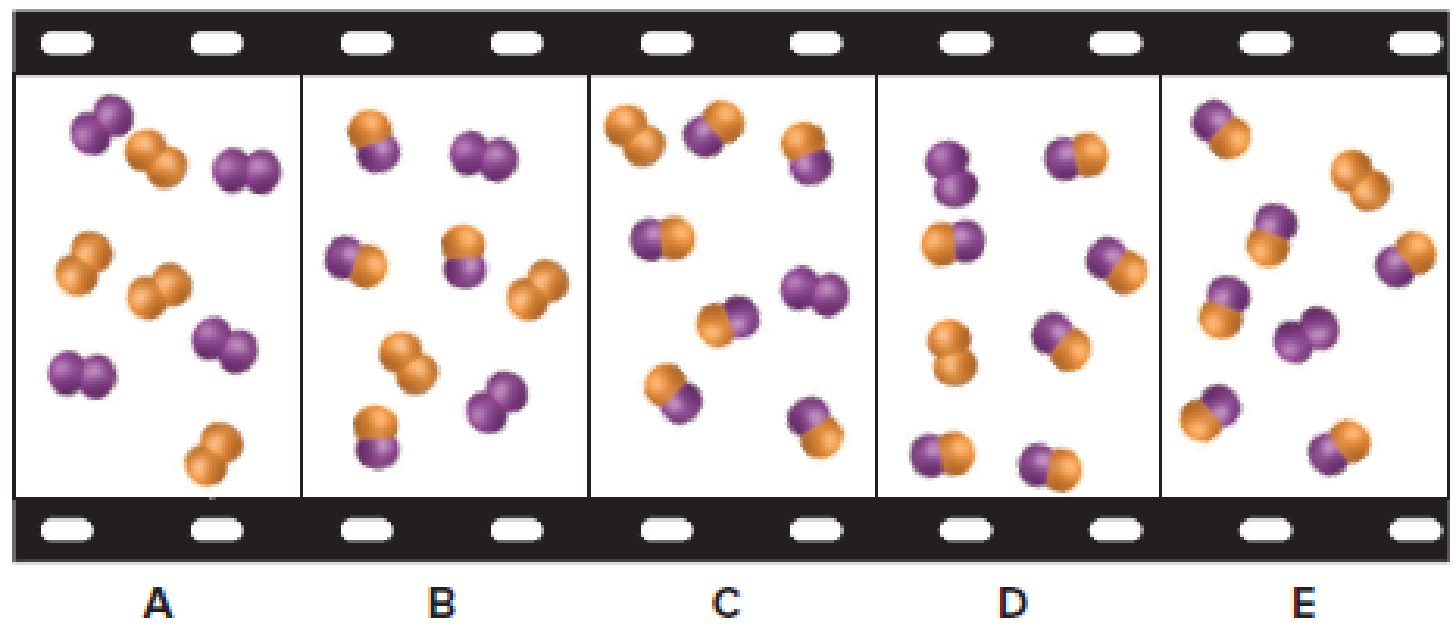
Figure 1
If
Concept Introduction:
Change in equilibrium due to temperature changes:
If the temperature is increased for the system, the equilibrium shifts away from the heat because of the reaction needs extra heat to use.
If the temperature is decreased for the system, the equilibrium shifts towards the heat because the heat needs to be produced to make up for the loss.
(e)
Explanation of Solution
Among the given five scenes, the scene that represents the mixture at a higher temperature is scene B. At higher temperatures, reaction shifts towards left forming more
(f)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
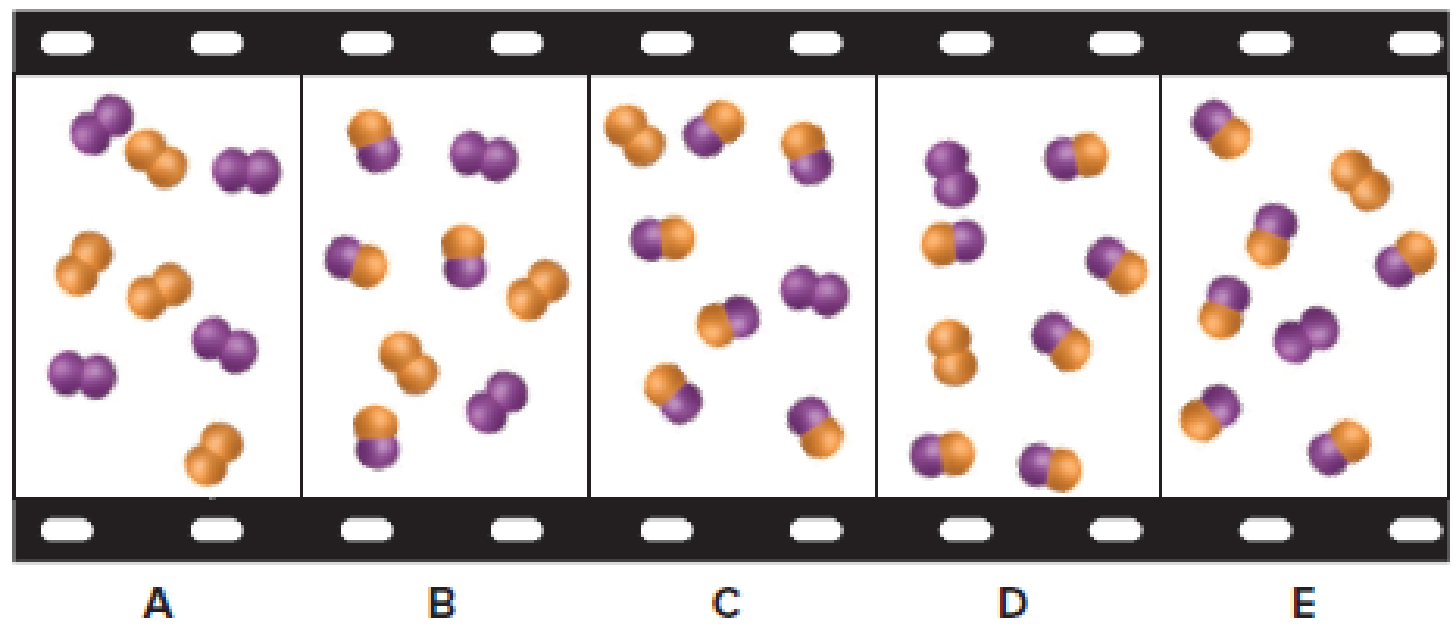
Figure 1
The scene that represents the mixture at a higher pressure (lower volume) has to be explained.
Concept Introduction:
Change in equilibrium due to pressure changes:
On increase in the system pressure, the equilibrium shifts towards fewer moles of gas, because, for gases,
On decrease in the system pressure, the equilibrium shifts towards more moles of gas, because, for gases,
(f)
Explanation of Solution
The given reaction is
The gaseous molecules in reactants are
Want to see more full solutions like this?
Chapter 17 Solutions
Chemistry: The Molecular Nature of Matter and Change - Standalone book
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





