
(a)
Interpretation:
It should be determined that the product obtained from the reaction of Ethyl butanoate with
Concept introduction:
Carboxylic compounds react with
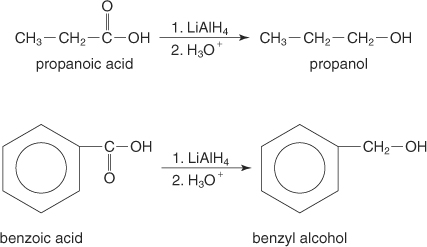
(b)
Interpretation:
It should be determined that the product obtained from the reaction of Benzoic acid with
Concept introduction:
Carboxylic compounds react with

(c)
Interpretation:
It should be determined that the product obtained from the reaction of Methyl benzoate with
Concept introduction:
Carboxylic compounds react with

(d)
Interpretation:
It should be determined that the product obtained from the reaction of Pentatonic acid
with
Concept introduction:
Carboxylic compounds react with

Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
ORGANIC CHEM(PKG) TEXT+MASTERING+SOL M
- Reaction of benzyl alcohol with ch3so2cl and pyridine and ch3ch2onaarrow_forwardWhat reagents would you use to convert methyl propanoate to the following compounds? a. isopropyl propanoate b. sodium propanoate c. N-ethylpropanamide d. propanoic acidarrow_forwardWhich could explain the stronger acidity of phenols compared to alcohols. Why? a.pi-electron delocalization b.steric effect c.hydrogen bonding d.hyperconjugationarrow_forward
- Explain the experimental procedure of the laboratory preparation of the synthesis of benzyl chloride from phenol Explain the chemistry behind the synthesis of benzyl chloride from phenolarrow_forwardWhat reaction converts benzoic acid to m-bromobenzoic acid? Alkylation Acylation Halogenation Hydrohalogenation Hydration Reduction Oxidation Nitration Sulfonationarrow_forwardDescribe how 3-methyl-1-phenyl-3-pentanol can be prepared from benzene. You can use any inorganic reagents and solvents, and any organic reagents provided they contain no more than two carbons.arrow_forward
- Why can’t 2-methyl-2-propanol be prepared by the reduction of a carbonyl compound?arrow_forwardThe result of the condensation reaction of aldehydes with phenols is: A) Arylmethane (auric) dye B) Indophenol dye C) azo dye D) Substituted benzophenonearrow_forwardIn the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. (a) Why are triphenylmethyl ethers so readily hydrolyzed by aqueous acid? (b) How might the structure of the triphenylmethyl group be modified to increase or decrease its acid sensitivity?arrow_forward
- Synthetize 3-phenyl-2-propenoic acid from benzaldehyde using whatever organic/inorganic reagents are needed. 3-phenyl-2-propenoic = 3-phenylacrylic acidarrow_forwardGive the products formed when benzaldehyde and benzoic acid are treated with the given reagents. 1 mole CH3OH, H+ LiAlH4 then H2O, H+arrow_forwardWhat is the reaction mechanism for formaldehyde and phenol under acidic conditions?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning




