
Concept explainers
Give IUPAC names for the following compounds:
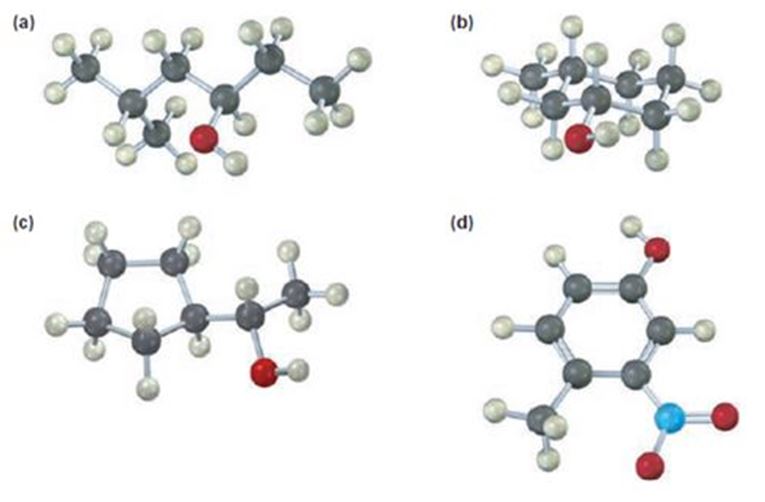
a)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Alcohols are named as derivatives of the parent alkane using the suffix–ol. The longest carbon chain containing the hydroxyl group is chosen and the parent name is derived by replacing the ending–e with–ol. The akane chain is numbered beginning at the end nearer to the hydroxyl group. The substituents are numbered according to their position on the chain. The name is written listing the substituents in the alphabetical order and indicating the position to which –OH is bonded.
If the compound is chiral then there can be two types of arrangements of the four groups attached to the chiral carbon, R and S. The four groups are arranged in the order of priority as 1,2,3 and 4 following sequence rules. The molecule is viewed by orienting the group of lowest priority (4) points away from the observer. If the arrangement of highest to second highest to third highest is clockwise the R configuration is assigned. If the arrangement is anticlockwise then S configurationis assigned.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
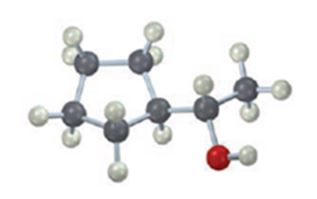
The compound given is
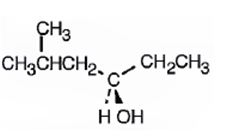
Its IUPAC name is (R) - 5 methylhexan-3-ol.
Explanation of Solution
The name of the compound indicates the presence of a six carbon straight chain with –OH on C3 and a methyl group on C5. Further the molecule is chiral. The groups –OH (highest ranking), -CH2CH(CH3)2(second highest ranking) and -CH2CH3(third highest ranking) are arranged around the chiral carbon in clockwise arrangement. Hence R configuration is assigned.
The compound given is

Its IUPAC name is (R) - 5 methylhexan-3-ol.
b)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
In naming cyclic alcohols the parent name is derived from the cycloalkene ring by replacing–e of the cycloalkene with–ol. The ring is numbered from the carbon with –OH in such a way that lowest number possible is given to the carbons with substituents.
A cis 1,3-disubstituted cycloalkane has the two substituent groups in a diequatorial arrangement.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
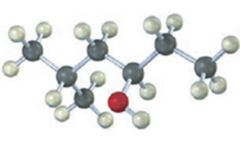
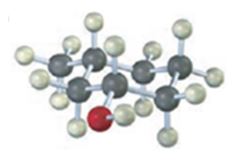
The compound given is
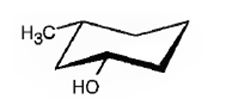
Its IUPAC name is cis-3-methylcyclohexanol.
Explanation of Solution
The structure shows that the compound contains a cyclohexane ring with a –OH and methyl groups in diequatorial positions on C1 and C3. Hence it is a cis-isomer.
The compound given is

Its IUPAC name is cis-3-methylcyclohexanol.
c)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Alcohols are named as derivatives of the parent alkane using the suffix–ol. The longest carbon chain containing the hydroxyl group is chosen and the parent name is derived by replacing the ending–e with–ol. He akane chain is numbered beginning at the end nearer to the hydroxyl group. The substituents are numbered according to their position on the chain. The name is written listing the substituents in the alphabetical order and indicating the position to which –OH is bonded.
If the compound is chiral then there can be two types of arrangements of the four groups attached to the chiral carbon, R and S. The four groups are arranged in the order of priority as 1,2,3 and 4 following sequence rules. The molecule is viewed by orienting the group of lowest priority (4) points away from the observer. If the arrangement of highest to second highest to third highest is clockwise the R configuration is assigned. If the arrangement is anticlockwise then S configurationis assigned.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
The compound given is
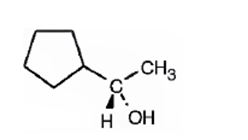
Its IUPAC name is (S)-1-cyclopentylethanol.
Explanation of Solution
The structure shows that the compound contains a two carbon straight chain with –OH and a cyclopentyl groups on C1.
The four groups, -OH(Highest priority), Cyclopentyl (second highest priority), methyl (third highest priority) attached to C1 are arranged anticlockwise when viewed away from hydrogen (fourth priority). Hence S configuration is assigned.
The compound given is

Its IUPAC name is (S)-1-cyclopentylethanol.
d)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Hydroxybenzenes are named as derivatives of phenol. If the ring contains other substituent also the ring is numbered from the carbon with –OH in such a way that lowest number possible is given to the carbon with the substituent. While writing the name the substituents are arranged in the alphabetical order.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
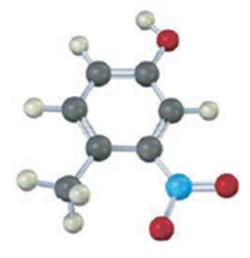
The compound given is.
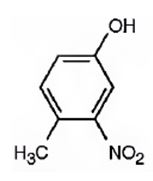
Its IUPAC name is 4-methyl-3-nitrophenol.
Explanation of Solution
The structure of the compound shows a benzene ring with –OH on C1, a nitro group on C3 and a methyl group on C4.
The compound given is.

Its IUPAC name is 4-methyl-3-nitrophenol.
Want to see more full solutions like this?
Chapter 17 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

