
Concept explainers
What carbonyl compounds might you start with to prepare the Following compounds by Grignard reaction? List all possibilities.
(a) 2-Methyl-2-propanol
(b) 1-Ethylcyclohexanol
(c) 3-Phenyl-3-pentanol
(d) 2-Phenyl-2-pentanol
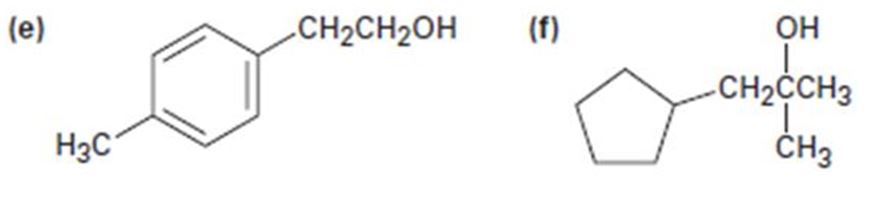
a) 2-Methyl-2-propanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol.
Answer to Problem 44AP
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
Explanation of Solution
A four carbon 30 alcohol is required. Hence a three carbon ketone (acetone) is treated with methylmagnesium bromide. In the case of esters two carbons will be provided by methylmagnesium bromide since esters require two molar equivalents of the reagent. Hence an ester of the two carbon acid (acetic acid) is chosen.
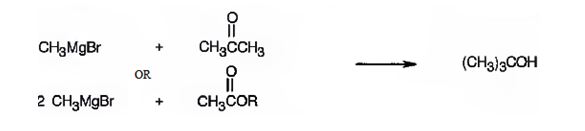
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
b) 1-Ethylcyclohexanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol.
Answer to Problem 44AP
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
Explanation of Solution
A six-membered cyclic 30 alcohol with ethyl group on C1 is required. Hence a six membered cyclic ketone (cyclohexanone) is treated with a two carbon Grignard reagent (ethylmagnesium bromide).
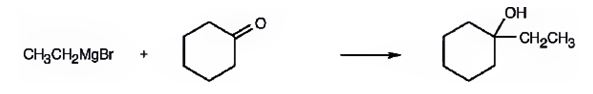
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
c) 3-Phenyl-3-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol.
Answer to Problem 44AP
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
Explanation of Solution
3-Phenyl-3-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C3. Hence an aromatic ketone (ethylphenyl ketone) is treated with ethylmagnesium bromide or the ester of benzoic acid is treated with two equivalents of ethylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with diethyl ketone.
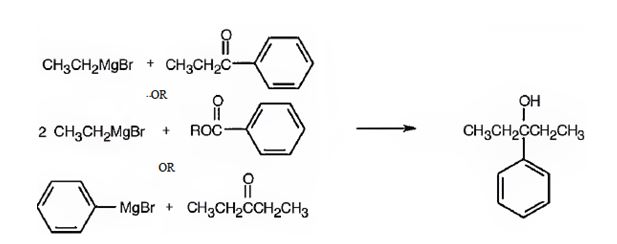
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
d) 2-Phenyl-2-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol.
Answer to Problem 44AP
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
Explanation of Solution
2-Phenyl-2-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C2. Hence an aromatic ketone like methylphenyl ketone is treated with propylmagnesium bromide or phenylpropyl ketone is treated methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with methylpropyl ketone.
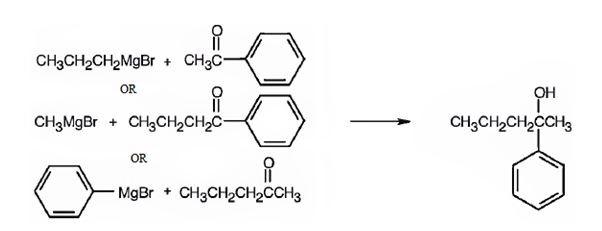
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
e)
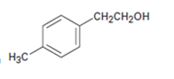
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-p-tolylethanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-(p-tolyl) ethanol.
Answer to Problem 44AP
2-(p-tolyl) ethanol can be prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
Explanation of Solution
2-(p-tolyl) ethanol is a 10 alcohol having a p-tolyl group attached to C2 of ethanol. Hence formaldehyde is required. The remaining part should come from the Grignard reagent. Hence formaldehyde is treated with p-tolylmethylmagnesium bromide.
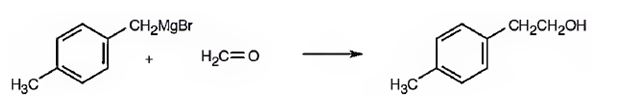
2-(p-tolyl) ethanol can prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
f)
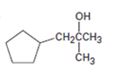
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Answer to Problem 44AP
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Explanation of Solution
1-Cyclopentyl-2-methyl-2-propanol is a 30 alcohol with a three carbon straight chain with a cyclopentyl group on C1 and –OH on C2. Hence cyclopentylmethyl methyl ketone is treated with methylmagnesium bromide or an ester of cyclopentylacetic acid is treated with two molar equivalents of methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence cyclopentylmethylmagnesium bromide is treated with acetone.
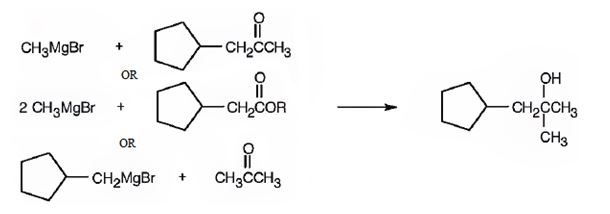
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Show the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2, and with diethylamine, (CH3CH2) 2NH.arrow_forwardDihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesulfonic acid, ArSO3H. Propose a mechanism for this conversion.arrow_forwardPropose a mechanism for the acid-catalyzed hydration of methylidenecyclohexane to give 1-methylcyclohexanol. Which step in your mechanism is rate-determining?arrow_forward
- ) How could you prepare cis-2,3-diethyloxirane from ethyne? (Show all reagents and intermediates).arrow_forwardPropose a synthesis route to prepare the following product from the given starting material. Note that more than one step reaction is required, and you must show all the reaction steps including preparation of the intermediates. (draw) 1 - propanol to 2-phenyl-2pentanolarrow_forwardPredict the products formed when cyclohexanone reacts with the following reagents.excess CH3OH, H+arrow_forward
- a)Write the SN2 reaction mechanism between iodobutane with sodium hydroxide, NaOH. b)Predict the products and show the SN1 reaction mechanism that occurs with 2-iodo-2- methylpropane in aqueous sodium hydroxide, NaOH.arrow_forwardHow would you prepare the following compounds from benzene, using a diazonium replacement reaction in your scheme? (a) p-Bromobenzoic acid (b) m-Bromobenzoic acid (c) m-Bromochlorobenzene (d) p-Methylbenzoic acid (e) 1, 2, 4-Tribromobenzenearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

