
a) PBr3
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with PBr3.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give
To give:
The products obtained when the alcohol shown is treated with PBr3.
Answer to Problem 22VC
The product obtained when the alcohol is treated with PBr3 is
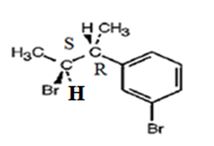
Explanation of Solution
The reaction of the alcohol with PBr3 is a bimolecular nucleophilic substitution (SN2) reaction. It takes place with inversion in configuration at the reaction centre to yield an alkyl bromide.
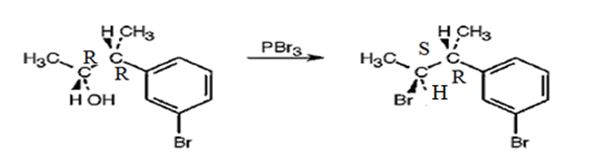
The product obtained when the alcohol is treated with PBr3 is

b) H2SO4
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with aqueous H2SO4.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with aqueous H2SO4.
Answer to Problem 22VC
The products obtained when the alcohol is treated with aqueous H2SO4 are two isomeric alkenes (E) and (Z). E isomer is obtained as the major product.
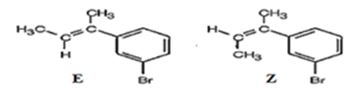
Explanation of Solution
The alcohol gets dehydrated when treated with aqueous H2SO4 yields the E and Z isomers of an alkene. The E isomer is more stable and obtained as the major product as the bulky groups are far away.
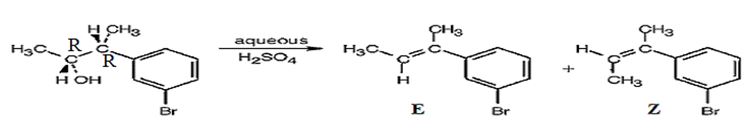
The products obtained when the alcohol is treated with aqueous H2SO4 isomeric alkenes (E) and (Z) are produced. E isomer is obtained as the major product.

c) SOCl2
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with SOCl2.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with SOCl2.
Answer to Problem 22VC
The product obtained when the alcohol is treated with SOCl2 is
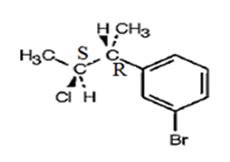
Explanation of Solution
The reaction of the alcohol with SOCl2 is a bimolecular nucleophilic substitution (SN2) reaction. It takes place with inversion in configuration at the reaction centre to yield an alkyl chloride.
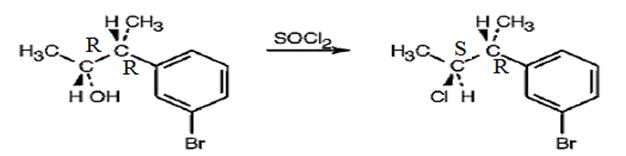
The product obtained when the alcohol is treated with SOCl2 is

d) Dess-Martin periodinate
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with Dess-Martin periodinate.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with Dess-Martin periodinate.
Answer to Problem 22VC
The product obtained when the alcohol is treated with Dess-Martin periodinate is
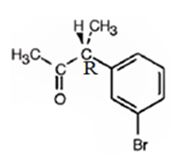
Explanation of Solution
The alcohol is a 20 in nature. Dess-Martin periodinate oxidizes it into a ketone.
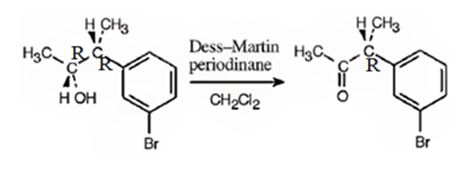
The product obtained when the alcohol is treated with Dess-Martin periodinate is

e) Br2, FeBr3
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated Br2, FeBr3.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated Br2, FeBr3.
Answer to Problem 22VC
The products obtained when the alcohol is treated with Br2, FeBr3 are
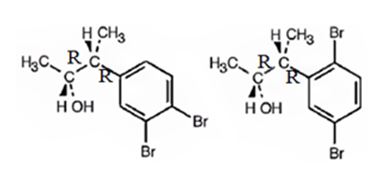
Explanation of Solution
The alcohol undergoes bromination in the benzene ring when treated with Br2, FeBr3. Both bromine and an alkyl group are attached to the ring. Both alkyl and Br are ortho and para directing, the alkyl is activating while the Br is deactivating. Hence during bromination the bromine enters into the ortho and para positions to yield the products.
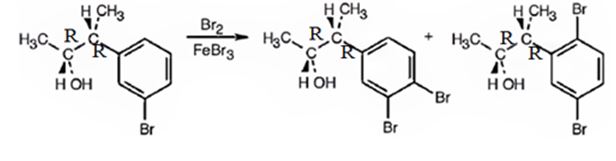
The products obtained when the alcohol is treated with Br2, FeBr3 are

Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- 16. When cis-1,2-cyclopentanediol reacts with acetone in dry HCl, compound M is formed. What do you expect the hydrolysis of compound M to be when subjected (i) to base; and, (ii) acid a. resistant to base; resistant to acid b. resistant to base; readily hydrolyzed by acid c. readily hydrolyzed by base; resistant to acid d. readily hydrolyzed by base; readily hydorlyzed by acid 17. Following (16), would you expect that a similar compound be formed if the reactant was trans-1,2-cyclopentanediol? Why is this so? a. Yes; hydroxyl groups are on opposite side b. No; hydroxyl groups are on opposite site c. Yes; hydroxyl groups are on the same side d. No; hydroxyl groups are on the same sidearrow_forwardRank the species below in order of increasing nucleophilicity in protic solvent. I. H2O II. CH3S— III. CH3COO— IV. t-BuO— I, II, IV, III I, III, II, IV I, III, IV, II I, II, III, IVarrow_forward1..Suggest mechanisms for the following reactions:arrow_forward
- Propose a plausible mechanism for the following process, called iodolactonizationarrow_forwardProvide a mechanism for the following isomerization in aqueous acid. Thank you in advance!arrow_forwardWhen N-bromosuccinimide is added to hex-1-ene in CCl4 and a sunlamp is shone on themixture, three products result.(a) Give the structures of these three products.(b) Propose a mechanism that accounts for the formation of these three productsarrow_forward
- The following transformation involves a series of four consecutive Heck reactions and the formation of the four-ring steroid nucleus (Section 26.4) as a racemic mixture. Propose structural formulas for the palladium-containing intermediates involved in this reaction.arrow_forwardOne step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forwardWhich of the following statements about terminal alkynes is FALSE?I I. A geminal dihalide is produced by the hydrohalogenation reaction.II. The proton in the terminal carbon is acidic but just slightly.III.They create an aldehyde when they react with H2O, H2SO4, and HgSO4.IV. A silver acetylide is formed after treatment with alcoholic AgNO3.arrow_forward
- Compelling evidence for the existence of a tetrahedral intermediate in nucleophilic acyl substitution was obtained in a series of elegant experiments carried out by Myron Bender in 1951. The key experiment was the reaction of aqueous−OH with ethyl benzoate (C6H5COOCH2CH3) labeled at the carbonyl oxygen with 18O. Bender did not allow the hydrolysis to go to completion, and then examined the presence of a label in the recovered starting material. He found that some of the recovered ethyl benzoate no longer contained a label at the carbonyl oxygen. With reference to the accepted mechanism of nucleophllic acyl substitution, explain how this provides evidence for a tetrahedral intermediate.arrow_forwardCompelling evidence for the existence of a tetrahedral intermediate in nucleophilic acyl substitution was obtained in a series of elegant experiments carried out by Myron Bender in 1951. The key experiment was the reaction of aqueous −OH with ethyl benzoate (C6H5COOCH2CH3) labeled at the carbonyl oxygen with 18O. Bender did not allow the hydrolysis to go to completion, and then examined the presence of a label in the recovered starting material. He found that some of the recovered ethyl benzoate no longer contained a label at the carbonyl oxygen. With reference to the accepted mechanism of nucleophilic acyl substitution, explain how this provides evidence for a tetrahedral intermediate.arrow_forward1. Predict and draw the structure of the product of the reaction of the following substrates with the given reducing agent. For c) indicate which components reacted. a) hexanal with NaBH4 b) 2-hexanone with LiAlH4 c) 2-hexanone mixed with hexanoic acid with i) NaBH4 ii) LiAlH4arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

