
a)
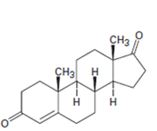
Interpretation:
Starting from testosterone how to prepare the compound shown is to be shown.
Concept introduction:
Pyridiniumchlorochromate in dichloromethane oxidizes 10 alcohols to
To show:
Starting from testosterone how to prepare the compound shown is to be stated.
b)
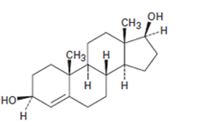
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
LiAlH4 in ether reduces unsaturated aldehydes, acids and esters to 10 alcohols. It reduces ketones to 20 alcohols. The double bond remains unaffected during the reduction.
To state:
Starting from testosterone how to prepare the compound shown.
c)
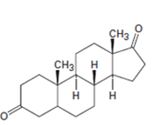
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
Pyridiniumchlorochromate in dichloromethane oxidizes 10 alcohols to aldehydes and 20 alcohols to ketones. H2, Pd/C can reduce the double bond in a compound without affecting aldehydic or keto group present in the compound.
To state:
Starting from testosterone how to prepare the compound shown.
d)
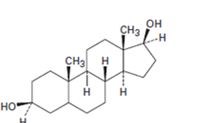
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
LiAlH4 in ether reduces unsaturated aldehydes, acids and esters to 10 alcohols. It reduces ketones to 20 alcohols. The double bond remains unaffected during the reduction. The double bond can be reduced using H2, Pd/C.
To state:
Starting from testosterone how to prepare the compound shown.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- How do you synthesize 3-oxocyclohexanecarboxylic acid from 2-cyclohexenone?give earrow_forwardShow the products you would expect to obtain from reaction of glyceryl Trioleate with the following reagents: (a) Excess Br2 in CH2Cl2 (b) H2/Pd (c) NaOH/H2O (d) O3, then Zn/CH3CO2H (e) LiAlH4, then H3O1 (f) CH3MgBr, then H3O1arrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forward
- Predict the product and propose a synthesis of p-bromoacetophenone from benzenearrow_forwardShow how you would synthesize the following compounds, starting with acetylene and any compounds containing nomore than four carbon atoms. pentanal, CH3CH2CH2CH2CHOarrow_forwardStarting with acetylene and 1-bromobutane as the only sources of carbon atoms, show how to synthesize the following. Q.) 6-Methyl-5-decanolarrow_forward
- Following is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors. Q.Why is it necessary to add the benzyl group, PhCH2—, as a blocking group in Step 1?arrow_forwardShow how you would accomplish the following syntheses.benzene ¡ p@methoxybenzaldehydearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

