
Concept explainers
a)
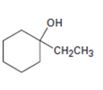
Interpretation:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how 1-ethylcyclohexanol can be prepared is to be stated.
Concept introduction:
An alicyclic 30 alcohol is to be prepared from benzene. For this purpose benzene has to be converted into an alicyclic
To state:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how to prepare 1-ethylcyclohexanol.
b)
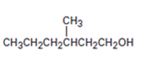
Interpretation:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how 3-methylhexan-1-ol can be prepared is to be given.
Concept introduction:
A six carbon straight chain alcohol with a methyl group on C3 is to be prepared from benzene. By ozonolysis, the benzene ring is broken to get the open chain dialdehyde glyoxal. To one of the aldehydic group in glyoxal, an isopentyl group can be introduced by treating with a suitable Grignard reagent. Upon heating the alcohol will eliminate water to yield an unsaturated
To give:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how to prepare 3-methylhexan-1-ol.
c)
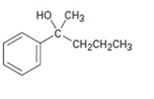
Interpretation:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how 2-methyl-2-phenyl-2 butanol can be prepared is to be given.
Concept introduction:
An
To give:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how to prepare .
d)
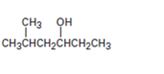
Interpretation:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how 5-methylhexan-3-ol can be prepared is to be given.
Concept introduction:
A six carbon straight chain aldehyde with a methyl group on C4 is to be prepared from benzene. By ozonolysis, the benzene ring is broken to get the open chain dialdehyde, maloaldehyde with three carbon atoms. To one of the aldehydic group in the dialdehyde, an isobutyl group can be introduced by treating with a suitable Grignard reagent to get an aldol. The aldehyde group in the aldol is reduced to an alkyl group to get the alcohol requireds.
To give:
Starting with benzene and using other alcohols of six or fewer carbons as the organic reagents how to prepare 3-methylhexan-1-ol.
Trending nowThis is a popular solution!

Chapter 17 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Using bromocyclohexane as a starting material, how could you synthesize the following compounds?arrow_forwardWrite the products of the following sequences of reactions. Refer to your reaction road-map to see how the combined reactions allow you to navigate between the different functional groups.arrow_forwardPropose a synthesis for (Z)-9-tricosene (muscalure), the sex pheromone for the common housefly (Musca domestica), starting with acetylene and haloalkanes as sources of carbon atoms.arrow_forward
- How would you synthesize the following 3-hexanone from butanenitrile using reagents from the table?arrow_forwardShow how you would synthesize the following compound, starting with benzene or toluene and any necessary reagents.arrow_forwardHow would you synthesize the following compound from acetylene and any alkyl halides with four or fewer carbons? More than one step may be required.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

