
Physics for Scientists and Engineers
10th Edition
ISBN: 9781337553278
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 11P
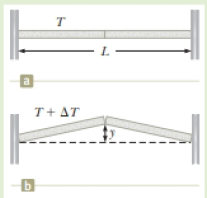
You are watching a new bridge being built near your house. You notice during the construction that two concrete spans of the bridge of total length Li = 250 m are placed end to end so that no room is allowed for expansion (Fig. P18.11a). In the opening storyline for this chapter, we talked about buckling sidewalks. The same thing will happen with spans on bridges if allowance is not made for expansion (Fig. P18.11b). You want to warn the construction crew about this dangerous situation, so you calculate the height y to which the spans will rise when they buckle in response to a temperature increase of ΔT = 20.0°C.
Figure P18.11
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A 40.0-g projectile is launched by the expansion of hot gas in an arrangement shown in Figure P12.4a. The cross-sectional area of the launch tube is 1.0 cm2, and the length that the projectile travels down the tube after starting from rest is 32 cm. As the gas expands, the pressure varies as shown in Figure P12.4b. The values for the initial pressure and volume are Pi = 11 x 105 Pa and Vi = 8.0 cm3 while the final values arePf = 1.0 x 105 Pa and Vf = 40.0 cm3. Friction between the projectile and the launch tube is negligible. (a) If the projectile is launched into a vacuum, what is the speed of the projectile as it leaves the launch tube? (b) If instead the projectile is launched into air at a pressure of 1.0 x 105 Pa, what fraction of the work done by the expanding gas in the tube is spent by the projectile pushing air out of the way as it proceeds down the tube?
During inhalation, a person's diaphragm and intercostal muscles contract, expanding the chest cavity and lowering the internal air pressure below ambient so that air flows in through the mouth and nose to the lungs. Suppose a person's lungs hold 1260 mL of air at a pressure of 1.00 atm. If they expand their chest cavity by 485 mL while keeping their nose and mouth closed so that no air is inhaled, what will be the air pressure in their lungs in atm? Assume the air temperature remains constant.
HINT
atm
During inhalation, a person’s diaphragm and intercostal muscles contract, expanding the chest cavity and lowering the internal air pressure below ambient so that air flows in through the mouth and nose to the lungs. Suppose a person’s lungs hold 1250 mL of air at a pressure of 1.00 atm. If the person expands the chest cavity by 525 mL while keeping the nose and mouth closed so that no air is inhaled, what will be the air pressure in the lungs in atm? Assume the air temperature remains constant.
Chapter 18 Solutions
Physics for Scientists and Engineers
Ch. 18.1 - Prob. 18.1QQCh. 18.3 - Consider the following pairs of materials. Which...Ch. 18.4 - If you are asked to make a very sensitive glass...Ch. 18.4 - Two spheres are made of the same metal and have...Ch. 18.5 - A common material for cushioning objects in...Ch. 18.5 - On a winter day, you turn on your furnace and the...Ch. 18 - Prob. 1PCh. 18 - Prob. 2PCh. 18 - Prob. 3PCh. 18 - Liquid nitrogen has a boiling point of 195.81C at...
Ch. 18 - Death Valley holds the record for the highest...Ch. 18 - Prob. 6PCh. 18 - A copper telephone wire has essentially no sag...Ch. 18 - A pair of eyeglass frames is made of epoxy...Ch. 18 - The Trans-Alaska pipeline is 1 300 km long,...Ch. 18 - A square hole 8.00 cm along each side is cut in a...Ch. 18 - You are watching a new bridge being built near...Ch. 18 - You are watching a new bridge being built near...Ch. 18 - At 20.0C, an aluminum ring has an inner diameter...Ch. 18 - Why is the following situation impossible? A thin...Ch. 18 - A volumetric flask made of Pyrex is calibrated at...Ch. 18 - Review. On a day that the temperature is 20.0C, a...Ch. 18 - Review. The Golden Gate Bridge in San Francisco...Ch. 18 - Your father and your younger brother are...Ch. 18 - An auditorium has dimensions 10.0 m 20.0 m 30.0...Ch. 18 - A container in the shape of a cube 10.0 cm on each...Ch. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - In state-of-the-art vacuum systems, pressures as...Ch. 18 - You have scored a great internship with NASA,...Ch. 18 - Review. The mass of a hot-air balloon and its...Ch. 18 - A room of volume V contains air having equivalent...Ch. 18 - Prob. 27PCh. 18 - You are applying for a position with a sea rescue...Ch. 18 - The pressure gauge on a cylinder of gas registers...Ch. 18 - A steel beam being used in the construction of a...Ch. 18 - Two metal bars are made of invar and a third bar...Ch. 18 - Why is the following situation impossible? An...Ch. 18 - A student measures the length of a brass rod with...Ch. 18 - The density of gasoline is 730 kg/m3 at 0C. Its...Ch. 18 - A liquid has a density . (a) Show that the...Ch. 18 - Prob. 36APCh. 18 - The rectangular plate shown in Figure P18.37 has...Ch. 18 - A bimetallic strip of length L is made of two...Ch. 18 - Prob. 39APCh. 18 - A vertical cylinder of cross-sectional area A is...Ch. 18 - Review. Consider an object with any one of the...Ch. 18 - Prob. 42APCh. 18 - Starting with Equation 18.11, show that the total...Ch. 18 - Review. A house roof is a perfectly flat plane...Ch. 18 - A 1.00-km steel railroad rail is fastened securely...Ch. 18 - Prob. 46CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P18.40). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find the height h in Figure P18.40. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder. Figure P18.40arrow_forwardA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P16.56). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find die height h in Figure P16.56. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder.arrow_forwardDuring inhalation, a person's diaphragm and intercostal muscles contract, expanding the chest cavity and lowering the internal air pressure below ambient so that air flows in through the mouth and nose to the lungs. Suppose a person's lungs hold 1240 mL of air at a pressure of 1.00 atm. If they expand their chest cavity by 515 mL while keeping their nose and mouth closed so that no air is inhaled, what will be the air pressure in their lungs in atm? Assume the air temperature remains constant.arrow_forward
- A deep-sea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of 1.01 X 105 N/m2. (a) What is the partial pressure of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of 2.00 X 106 N/m2, what percent oxygen should it be to have the same oxygen partial pressure as at sea level?arrow_forwardA pressure versus volume (pv) diagram for a system is shown in the figure. The arrows of the curve indicate the direction of the process, and the points of interest are labeled. The values for the points in the diagram are shown in the table. Volume (m3) Pressure (Pa) v0=27.4 p0=1.00×104 v1=19.3 p1=1.00×104 v2=16.0 p2=4.92×103 v3=13.3 p3=4.92×103 v4=13.3 p4=3.20×103 v5=7.51 p5=1.00×103 Calculate the amount of work done on the system from 0–2 (W02) and then for the entire curve from 0–5 (W05).arrow_forwardA 40.0-g projectile is launched by the expansion of hot gas in an arrangement shown in Figure P12.4a. The cross sectional area of the launch tube is 1.0 cm2, and the length that the projectile travels down the tube after starting from rest is 52 cm. As the gas expands, the pressure varies as shown in Figure P12.4b. The values for the initial pressure and volume are P1 = 11 105 Pa and Vi = 8.0 cm3 while the final values are Pf = 1.0 105 Pa and Vf = 8.0 cm3. Friction between the projectile and the launch tube is negligible, (a) If the projectile is launched into a vacuum, what is the speed of the projectile as it leaves the launch tube? (b) If instead the projectile is launched into air at a pressure of 1.0 105 Pa. what fraction of the work done by the expanding gas in the tube is spent by the projectile pushing air out of the way as it proceeds down tile tube?arrow_forward
- A gas is in a container of volume V0 at pressure P0. It is being pumped out of the container by a piston pump. Each stroke of the piston removes a volume Vs through valve A and then pushes the air out through valve B as shown in Figure P19.74. Derive an expression that relates the pressure Pn of the remaining gas to the number of strokes n that have been applied to the container. FIGURE P19.74arrow_forwardA deep sea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of 1.01 ✕ 105 N/m2. (a) What is the partial pressure (in N/m2) of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of 1.50 ✕ 106 N/m2, what percent oxygen should it be to have the same oxygen partial pressure as at sea level?arrow_forwardA flanged bolt coupling consists of ten steel 1/2-in.-diameter bolts spaced evenly around a bolt circle 14 in. in diameter. Determine the torque capacity of the coupling if the allowable shearing in the bolts is 6000 psi.arrow_forward
- The internal air pressure of a fridge reduces after closing its door due to cooling of air. If the temperature of all the trapped air falls from 30 deg Celcius to 5.0 deg Celcius after closing the fridge door, what is the magnitude of the resulting net force on the door? Take the volume of trapped air to be 0.30 m3, take the fridge to be perfectly sealed, and take the door area that is in contact with the trapped air to be 0.60 m2?arrow_forward1.88 moles of an ideal gas are placed in a container whose volume is 4.88 x10−3 m3. The absolute pressure of the gas is 6.3 x105 Pa. What is the average translational kinetic energy of a molecule of the gas?arrow_forwardAtmospheric pressure atop Mt. Everest is 4 2 3.30x10 N/m . (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percent oxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is 1.01 × 10^5 N/m^2 ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY