
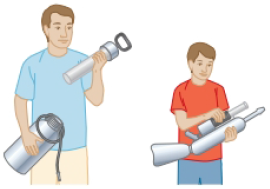
Your father and your younger brother are confronted with the same puzzle. Your father’s garden sprayer and your brother’s water cannon both have tanks with a capacity of 5.00 L (Fig. P18.18). Your father puts a negligible amount of concentrated fertilizer into his tank. They both pour in 4.00 L of water and seal up their tanks, so the tanks also contain air at atmospheric pressure. Next, each uses a hand-operated pump to inject more air until the absolute pressure in the tank reaches 2.40 atm. Now each uses his device to spray out water—not air—until the stream becomes feeble, which it does when the pressure in the tank reaches 1.20 atm. To accomplish spraying out all the water, each finds he must pump up the tank three times. Here is the puzzle: most of the water sprays out after the second pumping. The first and the third pumping-up processes seem just as difficult as the second but result in a much smaller amount of water coming out. Account for this phenomenon.
Figure P18.18
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Physics for Scientists and Engineers
- A gas is in a container of volume V0 at pressure P0. It is being pumped out of the container by a piston pump. Each stroke of the piston removes a volume Vs through valve A and then pushes the air out through valve B as shown in Figure P19.74. Derive an expression that relates the pressure Pn of the remaining gas to the number of strokes n that have been applied to the container. FIGURE P19.74arrow_forwardRefer to Problem 16 and Figure P14.16. A hydrometer is to be constructed with a cylindrical floating rod. Nine fiduciary marks are to be placed along the rod to indicate densities of 0.98 g/cm3, 1.00 g/cm3, 1.02 g/cm3, 1.01 g/cm3, 1.14 g/cm3. The row of marks is to start 0.200 cm from the top end of the rod and end 1.80 cm from the top end. (a) What is the required length of the rod? (b) What must be its average density? (c) Should the marks be equally spaced? Explain your answer.arrow_forwardWhich of the following statements is not correct?a. The faster a fluid flows, the lesser the pressure it exerts.b. The faster a fluid flows, the greater the pressure it exerts.c. The slower a fluid flows, the greater the pressure it exerts.d. The slower a fluid flows, the lesser the pressure it exerts.arrow_forward
- During inhalation, a person's diaphragm and intercostal muscles contract, expanding the chest cavity and lowering the internal air pressure below ambient so that air flows in through the mouth and nose to the lungs. Suppose a person's lungs hold 1260 mL of air at a pressure of 1.00 atm. If they expand their chest cavity by 485 mL while keeping their nose and mouth closed so that no air is inhaled, what will be the air pressure in their lungs in atm? Assume the air temperature remains constant. HINT atmarrow_forwardA gas flows through a square conduit. At one point along the conduit, the conduit sides are 0.100 m. the velocity it 7.55 m/s, and the gas's mass density is (for its particular pressure and temperature) 1.09 kg/m3. At a second point, the conduit sides are 0.250 m, and the velocity is 2.02 m/s. Find the mass flow rate of the gas and its mass density at the second point.arrow_forwardIn a dry-pipe sprinkler valve, the air seat typically has a surface area on which the air pressure acts six times as large as the water seat upon which the water pressure acts. If the water pressure is 105 psi, what air pressure is required to attain a state of equilibrium? answer should be in ( psi )arrow_forward
- A sphygmomanometer is a device used to measure blood pressure, typically consisting of an inflatable cuff and a manometer used to measure air pressure in the cuff. In a mercury sphygmomanometer, blood pressure is related to the difference in heights between two columns of mercury.The mercury sphygmomanometer shown in Figure P9.15 contains air at the cuff pressure P. The difference in mercury heights between the left tube and the right tube is h = 115 mmHg = 0.115 m, a normal systolic reading. What is the gauge systolic bloodpressure Pgauge in pascals? The density of mercury is p = 13.6 × 103 kg/m3 and the ambient pressure is P0 = 1.01 × 105 Pa. Figure P9.15arrow_forwardA deep-sea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dryair contains 20.9% oxygen and has a total pressure of 1.01×105 N/m2. (a) What is the partial pressure of oxygenat sea level? (b) If the diver breathes a gas mixture at a pressure of 2.00×106 N/m2, what percent oxygen shouldit be to have the same oxygen partial pressure as at sea level?arrow_forwardAtmospheric pressure atop Mt. Everest is 3.30 × 104 N/m2. (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percentoxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is 1.01 × 105 N/m2. (c) One of the most severe problems for those climbing very high mountains is the extreme drying of breathing passages. Why does this drying occur?arrow_forward
- I've posted the below question earlier, and I received the answer. This is the question: A J-shaped tube is filled with air at 760 Torr and 22 °C. The long arm is closed off at the top and is 100.0 cm long; the short arm is 40.00 cm high. Mercury is poured through a funnel into the open end. When the mercury spills over the top of the short arm, what is the pressure on the trapped air? Let h be the length of mercury in the long arm. I need further explanation of one step: - when P2 is obtained in the short arm, the equation is 40-h+P1 1. How did we get this equation? 2. the short arm does not contain any air, why do we create an equation of P2 in the short arm? Please clarify these concerns. I have received the answer shown in the attached picture but it is not correct.arrow_forwardAtmospheric pressure atop Mt. Everest is 4 2 3.30x10 N/m . (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percent oxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is 1.01 × 10^5 N/m^2 ?arrow_forwardWeek 4 #10 A scuba diver is in fresh water has an air tank with a volume of 0.0100 m3. The air in the tank is initially at a pressure of 1.00 × 107 Pa. Assume that the diver breathes 0.500 L/s of air. Density of fresh water is 1.00 × 103 kg/m3. How long will the tank last at depths of 4.80 m? _____minarrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning




