
(a)
Interpretation:
A
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an
Answer to Problem 11ST
Alpha emission of
Explanation of Solution
Alpha particle emission releases one alpha particle whose mass number decreases by
![]()
Figure 1
Radioactive alpha decay of
(b)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Beta emission of
Explanation of Solution
Beta emission decays into a proton and releases an electron or the atomic number increases by
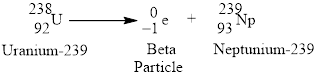
Figure 2
Radioactive beta decay of
(c)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Positron emission of
Explanation of Solution
In positron emission, an electron is released and its charge is
![]()
Figure 3
Radioactive positron electron emission of
(d)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Electron capture of
Explanation of Solution
In electron capture decay, element accepts electron and results in atomic number decreased by 1 and mass number remains same. The electron capture of
![]()
Figure 4
Radioactive alpha decay of
Want to see more full solutions like this?
Chapter 18 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





