
Concept explainers
(a)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
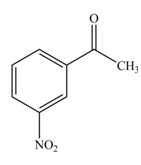
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus, it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
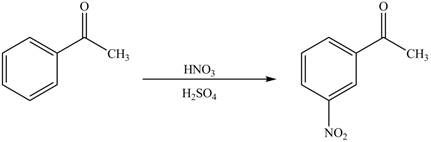
Figure 1
The product formed by the reaction of given compound with
(b)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
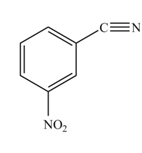
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
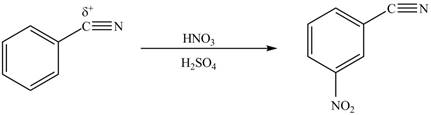
Figure 2
The product formed by the reaction of given compound with
(c)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
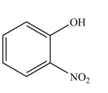
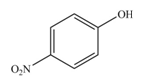
The reaction occurs faster because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para position.
The substituent present in the given compound is electron donating group. Thus, it directs the electrophile to ortho and para position and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
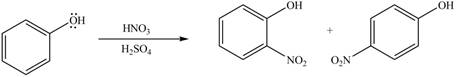
Figure 3
The product formed by the reaction of given compound with
(d)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The products formed by the reaction of given compound with
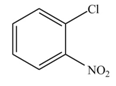
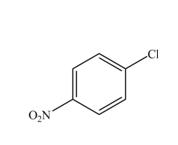
The reaction occurs slower benzene ring because benzene ring contains deactivating group.
Explanation of Solution
The substituent present in the given compound is
Among these two cases, mesomeric effect predominates over inductive effect. Hence, chlorine on benzene ring acts as releasing group but deactivates the benzene ring due to its
Thus, it directs the electrophile to ortho and para position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
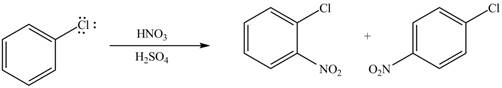
Figure 4
The product formed by the reaction of given compound with
(e)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The products formed by the reaction of given compound with
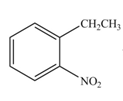
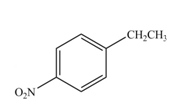
The reaction occurs higher because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para positions.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to ortho and para positions and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
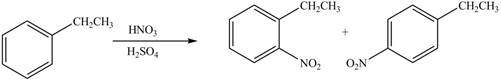
Figure 5
The product formed by the reaction of given compound with
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- Draw the carbon containing products of the following reaction: Reactant = 2-bromo-2-methyloctane Reagent = CN minus, room temperaturearrow_forwardWhat ester is formed when each carboxylic acid is treated with ethanol (CH 3CH 2OH) in the presence of H 2SO 4?arrow_forwardDraw the carbonyl products formed when each alcohol is oxidized with K 2Cr 2O 7.arrow_forward
- Draw the products formed when phenol (C6H5OH) is treated with following set of reagents. [1] CH3CH2Cl, AlCl3; [2] Br2, hνarrow_forwardWhat product is formed when benzene is treated with each organic halide in the presence of AlCl3?arrow_forwardDraw the products formed when phenol (C6H5OH) is treated with following set of reagents. [1] (CH3CH2)2CHCOCl, AlCl3; [2] Zn(Hg), HClarrow_forward
- Draw the structure of the major products if the given compound reacts with O3, then Zn, acetic acid.arrow_forward8 What ester is formed when each carboxylic acid is treated with 2-propanol [(CH 3) 2CHOH] in the presence of H 2SO 4?arrow_forwardDraw the organic products formed when each alkyne is treated with two equivalents of HBr.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


