
Concept explainers
(a)
Interpretation:
The product on reaction of
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 18.46AP
No product is formed on reaction of
Explanation of Solution
The reaction of
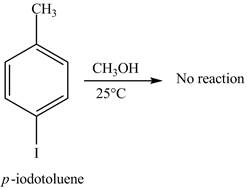
Figure 1
Aryl iodides cannot undergo nucleophilic substitution reaction. Aryl iodides neither undergo
There is no product formed on reaction of
(b)
Interpretation:
The product on reaction of
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as nucleophile. In nucleophilc acyl substitution reaction, nucleophile takes the position of leaving group.
Answer to Problem 18.46AP
No product is formed on reaction of
Explanation of Solution
The reaction of
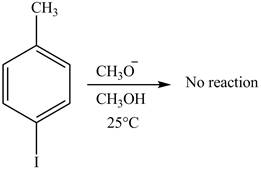
Figure 2
Aryl iodides cannot undergo nucleophilic substitution reaction. Aryl iodides neither undergo
There is no product formed on reaction of
(c)
Interpretation:
The product on reaction of
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as nucleophile. In nucleophilc acyl substitution reaction, nucleophile takes the position of leaving group.
Answer to Problem 18.46AP
No product is formed on reaction of
Explanation of Solution
The product on reaction of
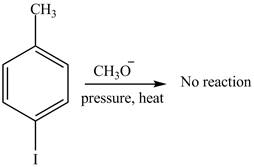
Figure 3
Aryl iodides cannot undergo nucleophilic substitution reaction. Aryl iodides neither undergo
There is no product formed on reaction of
(d)
Interpretation:
The product on reaction of
Concept introduction:
Grignard reagents are
Answer to Problem 18.46AP
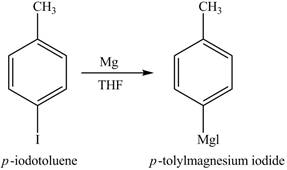
The product on reaction of
Explanation of Solution
The reaction of
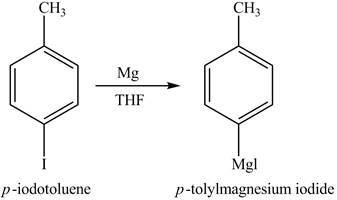
Figure 4
In the above reaction, magnesium gets inserted in the carbon-halogen bond to form a Grignard reagent. THF is used as the reaction should be done in anhydrous and inert condition. Therefore, the product formed on reaction of
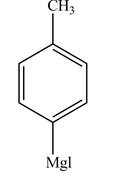
Figure 5
The reaction of
(e)
Interpretation:
The product on reaction of the product formed in part (d) with
Concept introduction:
Stille reaction is an example of coupling reaction. In Stille reaction, the triflate reacts with trimethylstannane in presence of
Answer to Problem 18.46AP
The product on reaction of the product formed in part (d) with
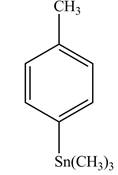
Explanation of Solution
The product formed in part (d) is shown below.
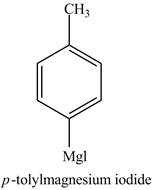
Figure 5
The reaction of the product formed in part (d) with
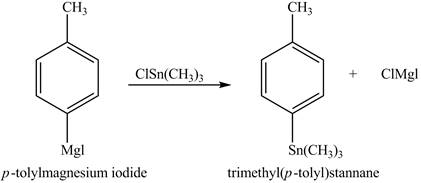
Figure 6
In the above reaction, a stannane compound is formed on reaction of a Grignard reagent with
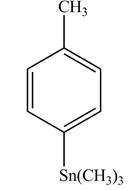
Figure 7
The product on reaction of the product formed in part (d) with
(f)
Interpretation:
The product on reaction of
Concept introduction:
Alkyl lithium is an organolithium reagent. It contains carbon-lithium bond. It is used in
Answer to Problem 18.46AP
The product on reaction of
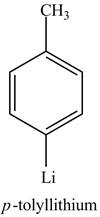
Explanation of Solution
The reaction of
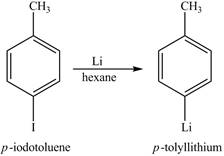
Figure 8
The above reaction is known as lithium-halogen exchange reaction. The reaction occurs under inert conditions. In this reaction, two moles of lithium react with
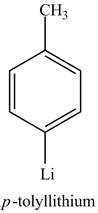
Figure 9
The product on reaction of
(g)
Interpretation:
The product on reaction of
Concept introduction:
The treatment of an organic halide with an
Answer to Problem 18.46AP
The product on reaction of
Explanation of Solution
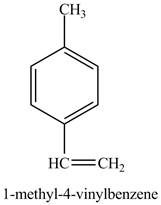
The reaction of
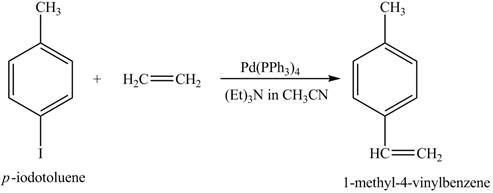
Figure 10
In the above reaction a coupled product is formed. The coupling takes place between ethene and
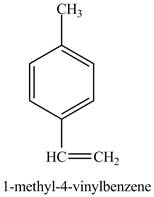
Figure 11
The product on reaction of
(h)
Interpretation:
The product on reaction of product of part (e) with phenyl triflate, excess
Concept introduction:
Stille reaction is an example of coupling reaction. In Stille reaction, the triflate reacts with trimethylstannane in presence of
Answer to Problem 18.46AP
The product on reaction of product of part (e) with phenyl triflate, excess
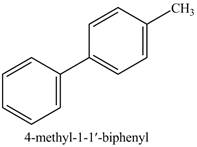
Explanation of Solution
The product formed in part (e) is shown below.
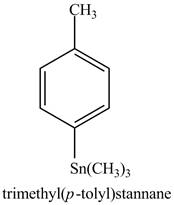
Figure 7
The reaction of above compound with phenyl triflate, excess
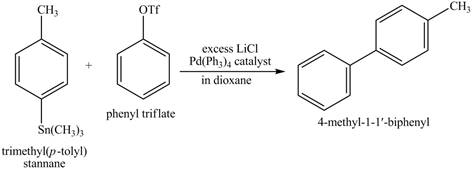
Figure 12
The above reaction is an example of Stille coupling reaction. In this reaction a triflate reacts with stannane compound in presence of
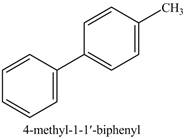
Figure 13
The product on reaction of product of part (e) with phenyl triflate, excess
(i)
Interpretation:
The product on reaction of
Concept introduction:
The Suzuki coupling reaction is a reaction in which an aryl or vinylic boronic acid is coupled to an aryl or vinylic iodide or bromide. It is a
Answer to Problem 18.46AP
The product on reaction of
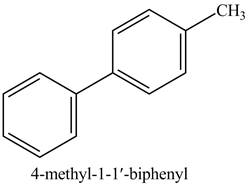
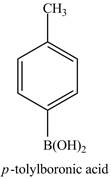
Explanation of Solution
The reaction of
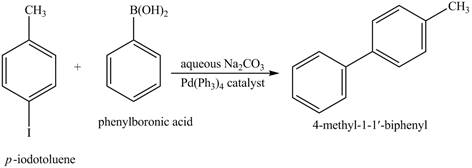
Figure 14
In the above reaction,
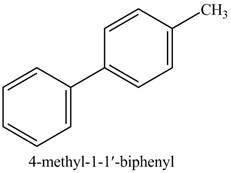
Figure 15
The product on reaction of
(j)
Interpretation:
The product on reaction of product of part (d) with
Concept introduction:
The Suzuki coupling reaction in which an aryl or vinylic boronic acid is coupled to an aryl or vinylic iodide or bromide. It is a
Answer to Problem 18.46AP
The product of part (d) with
Explanation of Solution
The product of part (d) is shown below.
Figure 5
The reaction of product of part (d) with
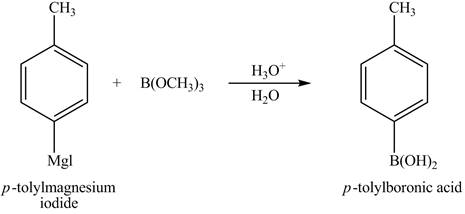
Figure 16
In the above reaction,
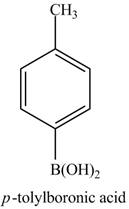
Figure 17
The product of part (d) with
(k)
Interpretation:
The product on reaction of product of part (j) with
Concept introduction:
The Suzuki coupling reaction in which an aryl or vinylic boronic acid is coupled to an aryl or vinylic iodide or bromide. It is a
Answer to Problem 18.46AP
The product on reaction of product of part (j) with
Explanation of Solution
The product of part (j) is shown below.
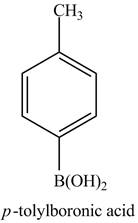
Figure 17
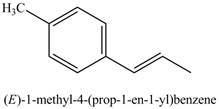
The reaction of product of part (j) with
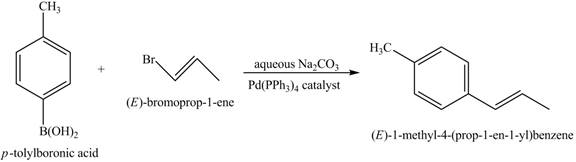
Figure 18
The above reaction is Suzuki coupling reaction. In this reaction,
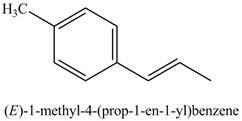
Figure 19
The product on reaction of product of part (j) with
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Compound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forwardWhen A is reacted with hot aqueous NaOH, a compound B of molecular formula C8H11NO is produced. With this information, write the correct structure of B and propose the reaction mechanism (step by step, with the correct use of arrows) to obtain B.arrow_forwardGive a proposal on the synthesis methods of CoSO4.7H2Oarrow_forward
- Draw a resonance structure of the acetonitrile anion, -: CH2CN, and account for the acidity of nitriles.arrow_forward5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardDefine the Mechanism - Conversion of Carboxylic Acids to Amides with DCCarrow_forward
- The treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formulaC5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forwardThe sex attractant of the housefly has the formula C23H46. When treated with warm potassium permanganate, this pheromone gives two products: CH3(CH2)12COOH and CH3(CH2)7COOH. Suggest a structure for this sex attractant. Explainwhich part of the structure is uncertainarrow_forward3 b and c) Give the productarrow_forward
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardCompound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct. Can be more than one answerarrow_forwardIsoprene has sometimes been used as a starting material in the laboratory synthesis of terpenes. In one such synthesis, the first step is the electrophilic addition of 2 mol of hydrogen bromide to isoprene to give 1,3-dibromo-3-methylbutane.Write a series of equations describing the mechanism of this reaction.arrow_forward
