
(a)
Interpretation:
The product of reaction of
Concept introduction:
The
Answer to Problem 18.47AP
The products formed on reaction of
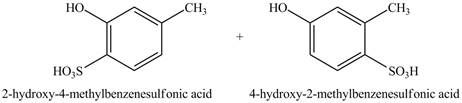
Explanation of Solution
The reaction of
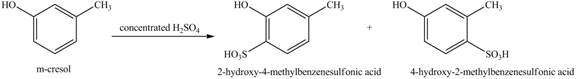
Figure 1
This reaction is known as electrophilic substitution reaction. The ortho, para directing power of hydroxyl group is greater than the methyl group, therefore, the sulfonic group is directed according to the position of hydroxyl group. Due to the presence of
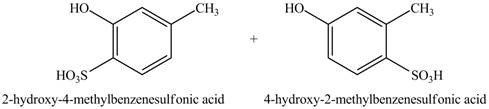
Figure 2
The products formed on reaction of
(b)
Interpretation:
The product on reaction of
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 18.47AP
The products formed on reaction of
Explanation of Solution
The reaction of
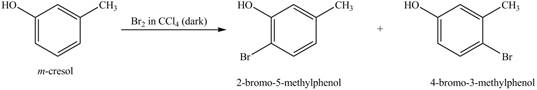
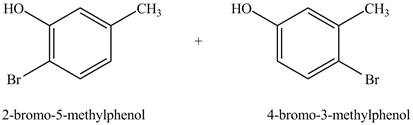
Figure 3
Under dark conditions, there is electrophilic substitution reaction. In this reaction, bromine will get substituted at the benzene ring. The position of the bromine depends on the hydroxyl group as it has more ortho, para directing power than methyl group. Therefore, the products formed on reaction of
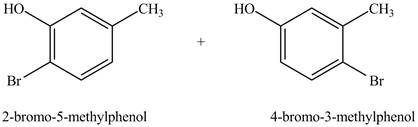
Figure 4
The products formed on reaction of
(c)
Interpretation:
The product on reaction of
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 18.47AP
The product on reaction of
Explanation of Solution
The reaction of
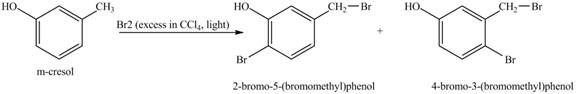
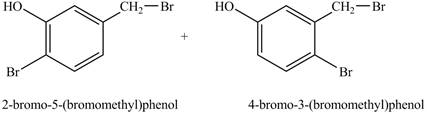
Figure 5
Under light conditions, there will be bromine radical formed and benzylic substitution will take place. Further, due to excess of bromine, bromine will get substituted at the benzene ring. The position of the bromine depends on the hydroxyl group as it has more ortho, para directing power than methyl group. Therefore, the products formed on reaction of
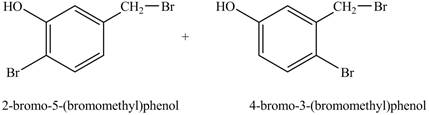
Figure 6
The product on reaction of
(d)
Interpretation:
The product on reaction of
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 18.47AP
No product is formed on reaction of
Explanation of Solution
The electron pair of oxygen of hydroxyl group is in conjugation with the phenyl ring. Due to this conjugation, it does not get protonated. Therefore, no reaction is taking place on adding dilute
There is no product formed on reaction of
(e)
Interpretation:
The product on reaction of
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 18.47AP
The product on reaction of
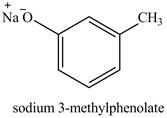
Explanation of Solution
The reaction of
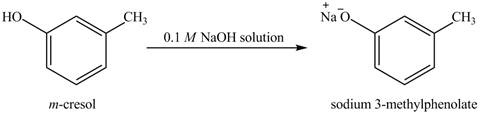
Figure 7
On adding sodium hydroxide, the hydroxyl group gets deprotonated to form a phenolate ion. Therefore, the product formed on reaction of
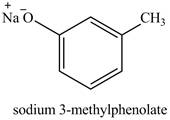
Figure 8
The product on reaction of
(f)
Interpretation:
The product on reaction of
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 18.47AP
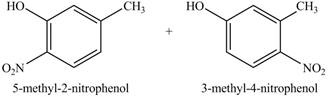
The product on reaction of
Explanation of Solution
The reaction of
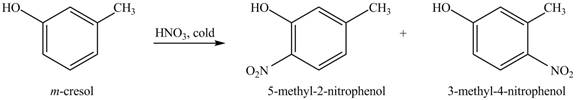
Figure 9
In this reaction, electrophilic substitution reaction occurs. Nitro will get substituted at the benzene ring. The position of the nitro group depends on the hydroxyl group as it has more ortho, para directing power than methyl group. Therefore, the products formed on reaction of
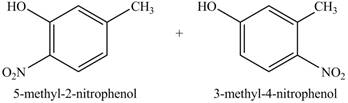
Figure 10
The product on reaction of
(g)
Interpretation:
The product on reaction of
Concept introduction:
The Friedel-Craft acylation is a type of electrophilic substitution reaction.
Answer to Problem 18.47AP
The products on reaction of
Explanation of Solution
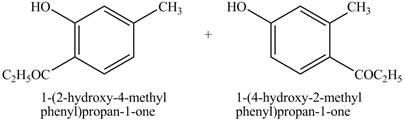
The reaction of
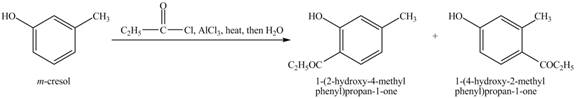
Figure 11
The above reaction is an example of Friedel Crafts Acylation reaction. In the above reaction,
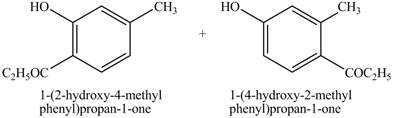
Figure 12
The products on reaction of
(h)
Interpretation:
The product formed on reaction of
Concept introduction:
Loss of electrons is classified as an oxidation reaction.
Answer to Problem 18.47AP
The product formed on reaction of
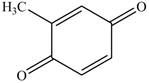
Explanation of Solution
The reaction of
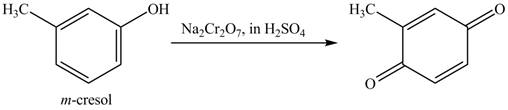
Figure 13
The above reaction is an oxidation reaction. The phenol group that has no substitution at para group gets oxidized to
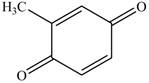
Figure 14
The product formed on reaction of
(i)
Interpretation:
The product on reaction of
Concept introduction:
Stille reaction is an example of coupling reaction. In Stille reaction, the triflate reacts with trimethylstannane in presence of
Answer to Problem 18.47AP
The product formed on reaction of
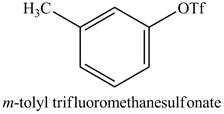
Explanation of Solution
The reaction of

Figure 15
In the above reaction, the triflic anhydride combines with the hydroxyl group of cresol to form
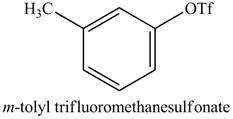
Figure 16
The product on reaction of
(j)
Interpretation:
The product on reaction of product of part (i) with
Concept introduction:
Stille reaction is an example of coupling reaction. In Stille reaction, the triflate reacts with trimethylstannane in presence of
Answer to Problem 18.47AP
The product on reaction of product of part (i) with
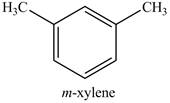
Explanation of Solution
The product of part (i) is shown below.
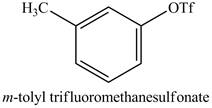
Figure 16
The reaction of product of part (i) with

Figure 17
In the above reaction,
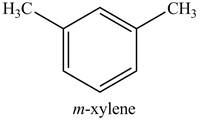
Figure 18
The product on reaction of product of part (i) with
(k)
Interpretation:
The product on reaction of product of part (i) with
Concept introduction:
The Suzuki coupling reaction is a reaction in which an aryl or vinylic boronic acid is coupled to an aryl or vinylic iodide or bromide. It is a
Answer to Problem 18.47AP
The product on reaction of product of part (i) with
Explanation of Solution
The product of part (i) is shown below.
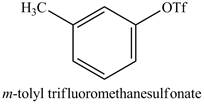
Figure 16
The reaction of product of part (i) with
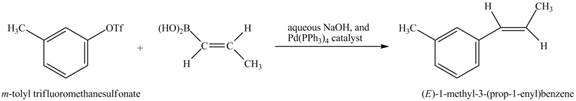
Figure 19
The above reaction is Suzuki coupling reaction. In this reaction,
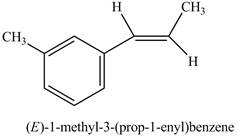
Figure 20
The product on reaction of product of part (i) with
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- 1. Write an equation for the oxidation of glucose by CuO if the compound is oxidizedcompletely to CO2 and H2O. 2. What can you conclude regarding the difference in degree of acidity and alkalinity between organic and inorganic compounds? ( sample substances are dilute hcl, dilute acetic acid, dilute nh4oh and aniline)arrow_forward3 b and c) Give the productarrow_forwardWhat can you conclude regarding the difference in degree of acidity and alkalinity between organic and inorganic compounds? ( sample substances are dilute hcl, dilute acetic acid, dilute nh4oh and aniline).arrow_forward
- 4b)Give the mechanisms for the following:arrow_forwardPropose a single synthesis method for cobalt(II) sulphate (CoSO4.7H2O) and describe in detail.arrow_forwardHow many grams of NaBH4 are needed to reduce 13.75 g of a ketone with molar mass 152.24 g/mol such that there are 1.53 equivalents of hydride?arrow_forward
- 3b)Give the mechanisms for the following transformations:arrow_forwardDetermine the direction of equilibrium when acetylene (HC≡CH) reacts with−NH2 in a proton transfer reaction ?arrow_forwardThe normal pH range for blood plasma is 7.35–7.45. Under these conditions, would you expect the carboxyl group of lactic acid (pKa 3.08) to exist primarily as a carboxyl group or as a carboxylic anion? Explain.arrow_forward
- Given the following reaction sequence: What are the reagents for reaction 3? A) NaNH2 excess followed by water B) sulfuric acid and heatarrow_forwardWill acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H2? Using pKa values given in Table 4.1, calculate Keq for this equilibrium.arrow_forwardFormaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative. When pure, formaldehyde trimerizes to give trioxane, C3H6O3, which, surprisingly enough, has no carbonyl groups. Only one monobromo derivative (C3H5BrO3) of trioxane is possible. Propose a structure for trioxane.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

