
Concept explainers
(a)
Interpretation:
The increasing order of acidity with the reason for the given compounds is to be stated.
Concept introduction:
Brønsted bases are those species which accept a proton. Base accepts a proton and forms conjugate acid. Brønsted acids are those species which donate a proton. Acid loses a proton and forms conjugate base. The acidity of the compound depends upon the stability of its conjugate base. The more stable the conjugate base, the more acidic the given compound. It also depends on the charge, inductive effects, the role of atom present.
Answer to Problem 18.49AP
The acidity of the given compounds in increasing order is shown below.
This is because of the conjugate base stability and resonance stabilization of benzene thiol.
Explanation of Solution
The acidity of the compound depends upon the stability of its conjugate base. The greater the stability of the conjugate base more acidic the given compound. The structure of the conjugate base of the given compounds is shown below.
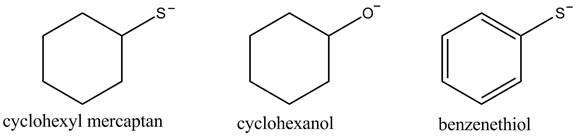
Figure 1
The conjugate base of benzene thiol is stabilized by the resonance that is why its acidity is greater among the given compounds. The cyclohexyl mercaptan contains sulfur atom which is larger than the oxygen atom, due to this it is more capable of diffusing negative charge. Therefore, the cyclohexyl mercaptan conjugate base is more stable than the cyclohexanol. This results in greater acidity of the cyclohexyl mercaptan compound.
The increasing order of acidity of the given compound is shown below.
The increasing order of acidity is
(b)
Interpretation:
The increasing order of acidity with the reason for the given compounds is to be stated.
Concept introduction:
Brønsted bases are those species which accept a proton. Base accepts a proton and forms conjugate acid. Brønsted acids are those species which donate a proton. Acid loses a proton and forms conjugate base. The acidity of the compound depends upon the stability of its conjugate base. The more stable the conjugate base, the more acidic the given compound. It also depends on the charge, inductive effects, the role of the atom present, resonance, orbitals.
Answer to Problem 18.49AP
The acidity of the given compounds in increasing order is shown below.
This is because of the conjugate base stability and resonance stabilization of phenol and
Explanation of Solution
The acidity of the compound depends upon the stability of its conjugate base. The greater the stability of the conjugate base more acidic the given compound. The structure of the conjugate base of the given compounds is shown below.
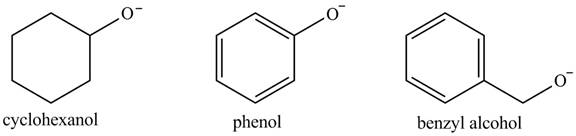
Figure 2
The conjugate base of phenoxide ion is stabilized by the resonance that is why its acidity is greater among the given compounds. The conjugate base of benzyl alcohol exerts
The increasing order of acidity is
(c)
Interpretation:
The increasing order of acidity with the reason for the given compounds is to be stated.
Concept introduction:
Brønsted bases are those species which accept a proton. Base accepts a proton and forms conjugate acid. Brønsted acids are those species which donate a proton. Acid loses a proton and forms conjugate base. The acidity of the compound depends upon the stability of its conjugate base. The more stable the conjugate base, the more acidic the given compound. It also depends on the charge, inductive effects, electronegativity, resonance, orbitals.
Answer to Problem 18.49AP
The acidity of the given compounds in increasing order is shown below.
This is because of the conjugate base stability and equivalent resonance stabilization of nitric acid.
Explanation of Solution
The acidity of the compound depends upon the stability of its conjugate base. The greater the stability of the conjugate base more acidic the given compound. The structure of the conjugate base of the given compounds is shown below.
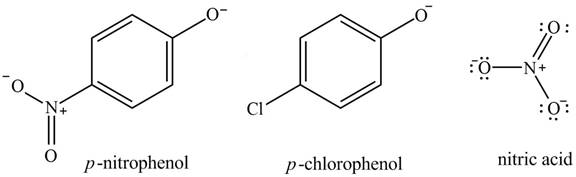
Figure 3
The equivalent resonance structures are more stable as compared to nonequivalent resonance structures. The nitric acid conjugate base shows equivalent resonance structures. Also, it is stabilized by the negative charge on the two oxygen atoms. It also exerts
The increasing order of acidity is
(d)
Interpretation:
The increasing order of acidity with the reason for the given compounds is to be stated.
Concept introduction:
Brønsted bases are those species which accept a proton. Base accepts a proton and forms conjugate acid. Brønsted acids are those species which donate a proton. Acid loses a proton and forms conjugate base. The acidity of the compound depends upon the stability of its conjugate base. The more stable the conjugate base, the more acidic the given compound. It also depends on the charge, inductive effects, electronegativity, resonance, orbitals.
Answer to Problem 18.49AP
The acidity of the given compounds in increasing order is shown below.
This is because of the conjugate base stability and resonance stabilization of
Explanation of Solution
The acidity of the compound depends upon the stability of its conjugate base. The greater the stability of the conjugate base more acidic the given compound. The structure of the conjugate base of the given compounds is shown below.
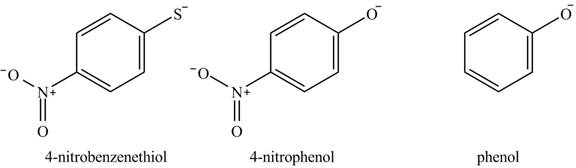
Figure 4
The conjugate base of
The increasing order of acidity is
(e)
Interpretation:
The increasing order of acidity with the reason for the given compounds is to be stated.
Concept introduction:
Brønsted bases are those species which accept a proton. Base accepts a proton and forms conjugate acid. Brønsted acids are those species which donate a proton. Acid loses a proton and forms conjugate base. The acidity of the compound depends upon the stability of its conjugate base. The more stable the conjugate base, the more acidic the given compound. It also depends on the charge, inductive effects, electronegativity, resonance, orbitals.
Answer to Problem 18.49AP
The acidity of the given compounds in increasing order is shown below.
This is because of the conjugate base stability,
Explanation of Solution
The acidity of the compound depends upon the stability of its conjugate base. The greater the stability of the conjugate base more acidic the given compound. The structure of the conjugate base of the given compounds is shown below.
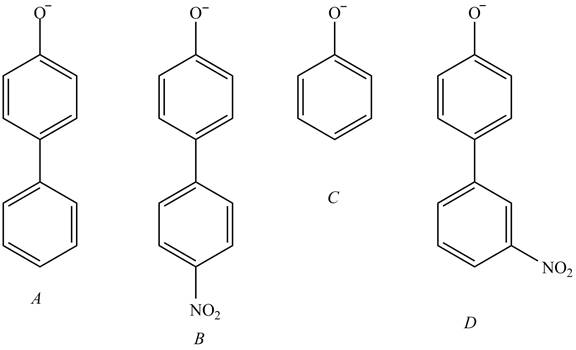
Figure 5
The conjugate base of the compound B stabilized by
The increasing order of acidity is
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenolarrow_forwardOrder the following in increasing acid strength and explain your reasoning. a. Benzoic Acid, b. 4-nitrobenzoic acid, c. 4-methylbenzoic acid, d. 4-methoxybenzoic acidarrow_forwardExplain this statement: Although 2-methoxyacetic acid (CH3OCH2COOH)is a stronger acid than acetic acid (CH3COOH), p-methoxybenzoic acid(CH3OC6H4COOH) is a weaker acid than benzoic acid (C6H5COOH).arrow_forward
- p-Nitrobenzyl alcohol is more acidic than benzyl alcohol but p-Methoxybenzyl alcohol is less acidic. Illustrate your explanation.arrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forwardRank each of the following sets of nitrogen bases in terms of basicity and explain your answerarrow_forward
- a. Compound X is benzene, Y is acetic anhydride acid. Complete the following scheme and determine Z! b. Determine which reagents except acetic acid anhydrides can replace Y!arrow_forwardStarting with the following compounds, outline a practical synthesis of 1-butanolarrow_forwardWhich is the stronger acid in each of the following pairs? Explain your reasoning. (a) Phenol or p-hydroxybenzaldehyde (b) m-Cyanophenol or p-cyanophenol (c) o-Fluorophenol or p-fluorophenolarrow_forward
- Write the chemical equation for the acid dissociation of acetaminophen, C8H9O2N. Write the Ka expression for the acid dissociation of acetaminophen.arrow_forwardWrite reactions of ethyl chloride with the following reagents: a. KOH, aqueous solution b. NaCNarrow_forwardSuggest how you would synthesize each compound, use cyclopentanone as one of the reagentsarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

