
(a)
Interpretation:
The mechanism and product for the given reactions, when they are catalyzed by the base, are to be drawn.
Concept introduction:
The carbonyl carbon is electrophilic in nature due to the electronegativity difference. The nucleophile adds the electrophilic carbon of the carbonyl group. The reaction follows a different mechanism for an acid and a base catalyst. When the reaction is catalyzed by the acid, the first step of the reaction is always protonation of the carbonyl oxygen, which increases the electrophilicity of the carbonyl carbon. For a base-catalyzed reaction, the first step is the deprotonation of the nucleophilic species to form the anion, which helps to enhance the nucleophilic character.
Answer to Problem 18.46P
- The mechanism for the first reaction, when it catalyzed by the base:
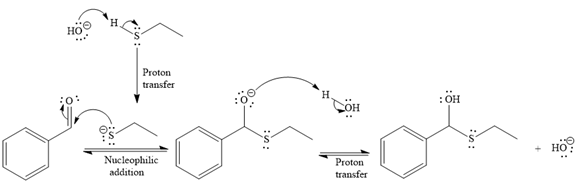
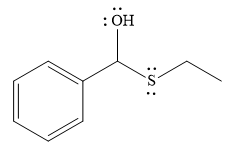
- The product for the first reaction, when it catalyzed by the base:
- The mechanism for the second reaction, when it catalyzed by the base:
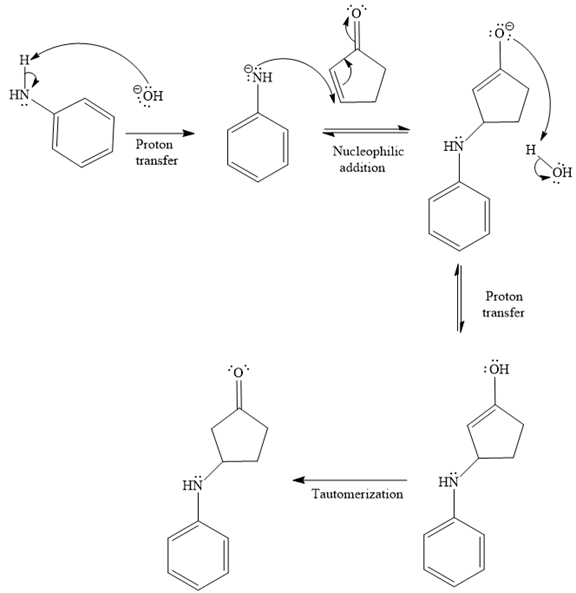
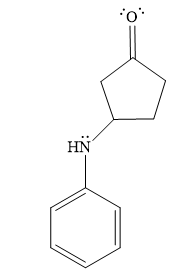
- The product for the second reaction, when it catalyzed by the base:
Explanation of Solution
The first given reaction is
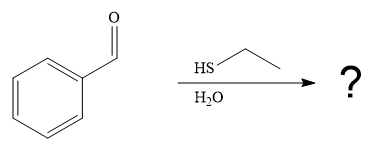
Under the basic condition, the base (
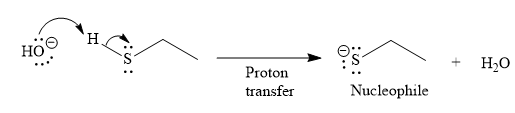
The nucleophile adds the electrophilic carbon of the given substrate, leading to form an alkoxide ion. Alkoxide ion then abstracts proton from the solvent, water, to form the uncharged product.
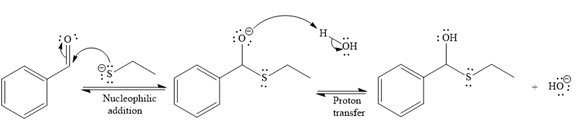
Thus, the product of the first reaction, when it catalyzed by the base, is

The second given reaction is
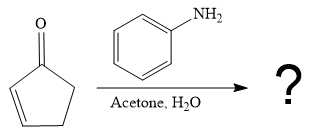
Under the basic condition, the base (
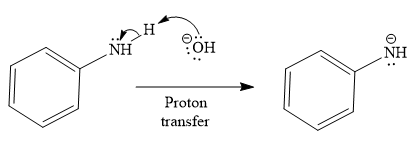
In the second step, the attack of the nucleophile (
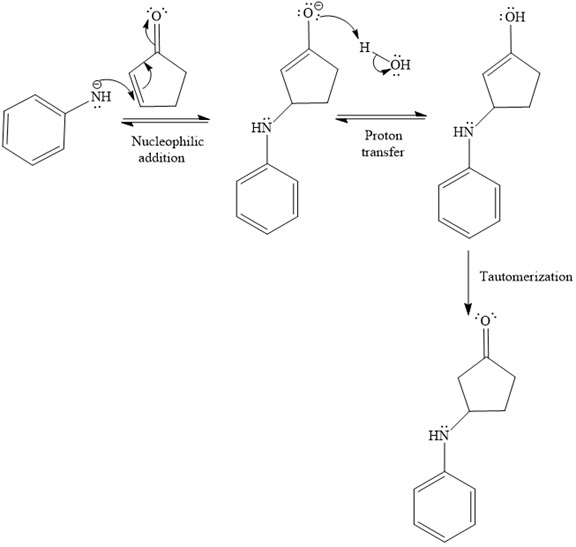
Thus, the product of the given reaction is

The mechanism and product for the given reaction are drawn on the basis of the reaction condition.
(b)
Interpretation:
The mechanism and product for the given reactions, when they are catalyzed by the acid, are to be drawn.
Concept introduction:
The carbonyl carbon is electrophilic in nature due to the electronegativity difference. The nucleophile adds the electrophilic carbon of the carbonyl group. The reaction follows a different mechanism for an acid and a base catalyst. When the reaction is catalyzed by the acid, the first step of the reaction is always protonation of the carbonyl carbon, which increases the electrophilicity of the carbonyl oxygen. For a base-catalyzed reaction, the first step is the deprotonation of the nucleophilic species to enhance the nucleophilic character.
Answer to Problem 18.46P
- The mechanism for the first given reaction, when it catalyzed by the acid:
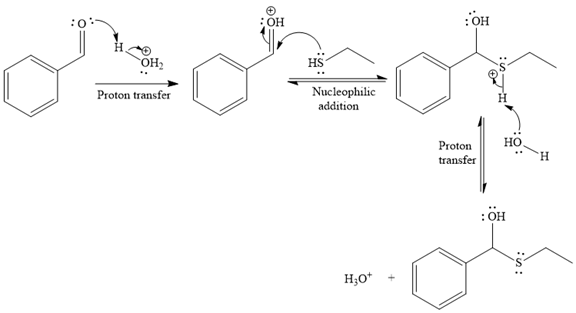
- The product for the first reaction, when it catalyzed by the acid:

- The mechanism for the second reaction, when it catalyzed by the acid:
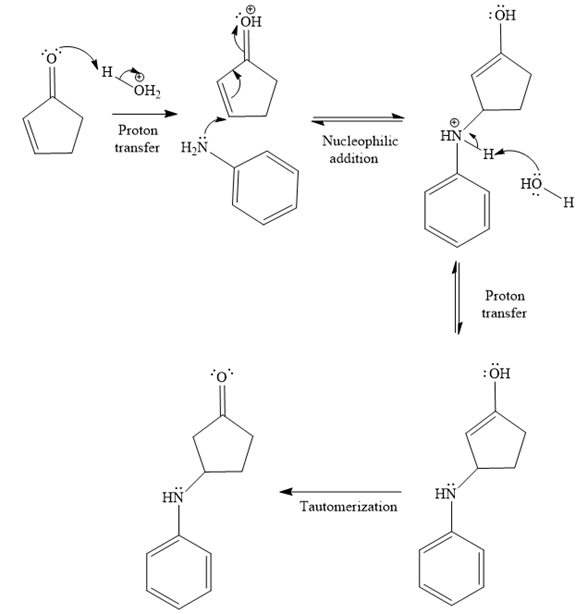
- The product for the second reaction, when it catalyzed by the acid:

Explanation of Solution
The first given reaction is

Under the acidic condition, the carbonyl oxygen of the given reactant (an
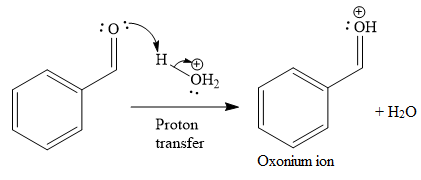
Then the nucleophile attack of (
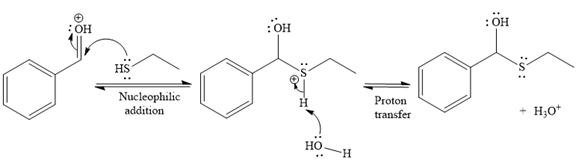
Thus, the product of the given reaction is

The second given reaction is

Under the acidic condition, the carbonyl oxygen of the given reactant (
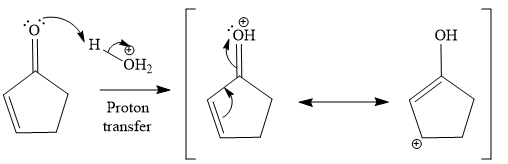
In the second step, the attack of the nucleophile (
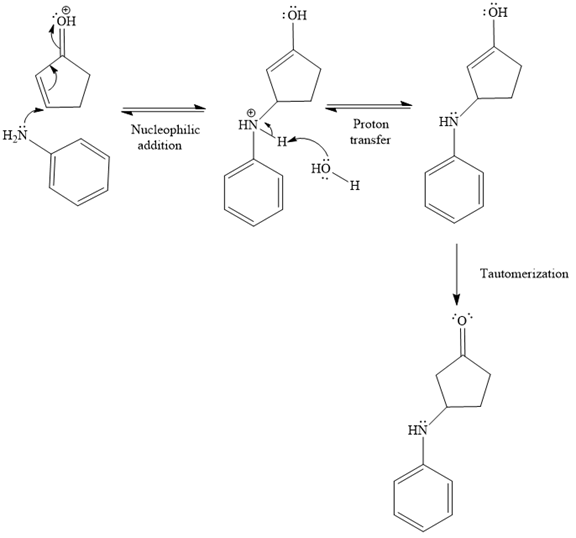
Thus, the product of the given reaction is

The mechanism and product for the given reaction are drawn on the basis of the reaction condition.
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





