
Concept explainers
(a)
Interpretation:
The synthesis of the given compound using the cyclopentanone as one of the reagents is to be suggested.
Concept introduction:
In aldol condensation, the
Answer to Problem 18.69P
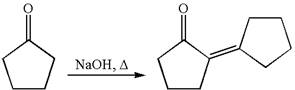
The synthesis of the given compound using cyclopentanone as one of the reagents is:
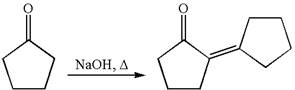
Explanation of Solution
The given compound is:
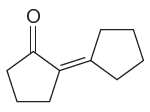
The given compound is a
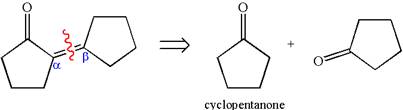
The precursor determined is cyclopentanone. Two molecules of cyclopentanone are used in the aldol condensation. Cyclopentanone, on reaction with a strong base, forms the given
The synthesis of the given compound is planned by identifying the precursor used with disconnection of
(b)
Interpretation:
The synthesis of the given compound using cyclopentanone as one of the reagents is to be suggested.
Concept introduction:
In aldol condensation, the aldehyde or ketone with
Answer to Problem 18.69P
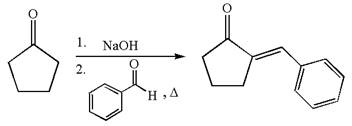
The synthesis of the given compound using cyclopentanone as one of the reagents is:
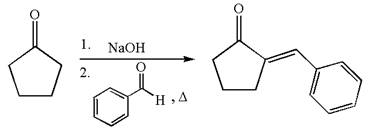
Explanation of Solution
The given compound is:
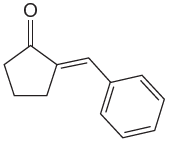
The given compound is
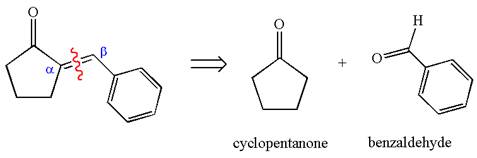
The precursors determined are cyclopentanone and benzaldehyde. As the benzaldehyde has no
The synthesis of the given compound is planned by identifying the precursor used with disconnection of
(c)
Interpretation:
The synthesis of the given compound using cyclopentanone as one of the reagents is to be suggested.
Concept introduction:
In aldol condensation the aldehyde or ketone with
Answer to Problem 18.69P
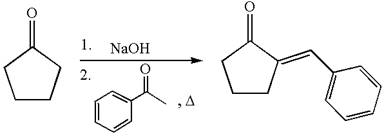
The synthesis of the given compound using cyclopentanone as one of the reagents is:
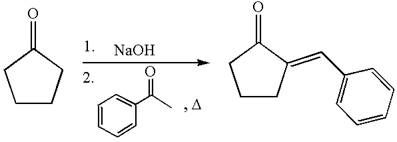
Explanation of Solution
The given compound is:
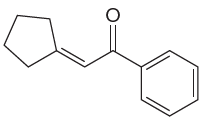
The given compound is
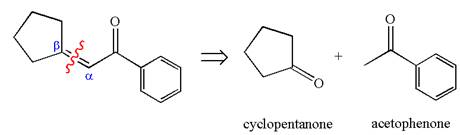
The precursors determined are the cyclopentanone and acetophenone. As the both ketones has
The synthesis of the given compound is planned by identifying the precursor used with disconnection of
(d)
Interpretation:
The synthesis of the given compound using cyclopentanone as one of the reagents is to be suggested.
Concept introduction:
In aldol condensation the aldehyde or ketone with
Instead of carbonyl compounds, the compounds with polar
Answer to Problem 18.69P
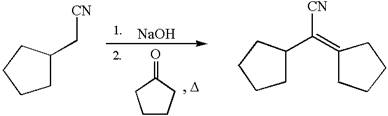
The synthesis of the given compound using cyclopentanone as one of the reagents is:
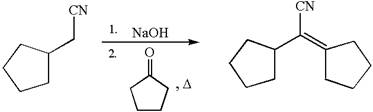
Explanation of Solution
The given compound is:
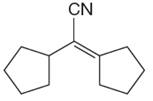
The given compound is
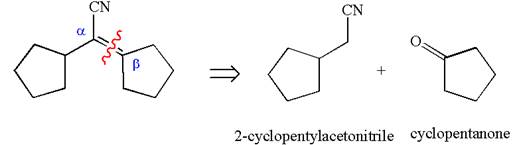
The precursors determined are cyclopentanone and nitrile compound. As the nucleophile adds to carbonyl, the nucleophile can be produced by the reaction of one of the nitrile with a strong base like hydroxide. The nucleophile then reacts with ketone and produces the given
The synthesis of the given compound is planned by identifying the precursor used with disconnection of
(e)
Interpretation:
The synthesis of the given compound using cyclopentanone as one of the reagents is to be suggested.
Concept introduction:
The Robinson annulations reaction is the addition of enolate to conjugated carbonyl followed by intramolecular aldol condensation. One of the reactant in Robinson annulations must be
Answer to Problem 18.69P
The synthesis of given compound using cyclopentanone as one of the reagents is:
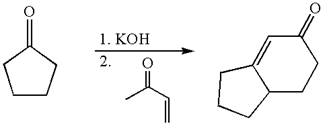
Explanation of Solution
The given compound is:
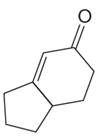
The given compound is
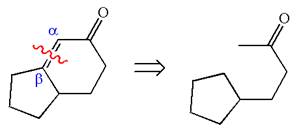
To get the precursor
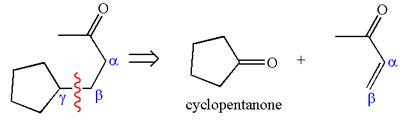
The precursors determined are the cyclopentanone and
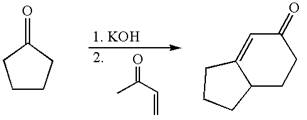
The synthesis of given compound is planned by identifying the precursor used with disconnection of
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Please predict the product of the following reaction, and draw the complete, detailed mechanism.arrow_forwardSolve correctly please. With explanation also. (Answer only if sure) What is the major product of the following reaction?arrow_forwardPlease answer question below, be very deatiled and draw out the mechanism reaction with arrowsarrow_forward
- (SYN) Show how you would synthesize each of these compounds, using butan-1-ol and benzene as your only sources of carbon.arrow_forward(SYN) Show how each of the following compounds can be synthesized from an acid chloride and either water, an alcohol, or an amine. For each reaction, provide the complete, detailed mechanism.arrow_forward(SYN) Show how to carry out the following conversion.Hint: Consider using a protecting group.arrow_forward
- Propose a mechanism for the reaction shown here, which takes place under conditions that favor anarrow_forwardPredict the major product of each of the reactions shown here and provide the complete, detailed mechanism.arrow_forwardTextbook problem: Suggest short series of reactions that would be expected to transform the material on the right into the desired product shown on left.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
