
Concept explainers
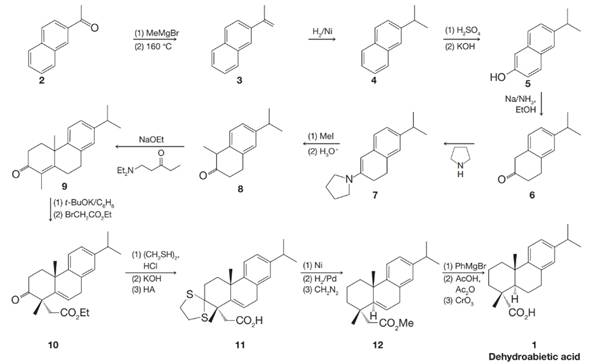
Dehydroabietic acid is a natural product isolated from Pinus palustris. It is structurally related to abietic acid, which comes from rosin. The synthesis of dehydroabietic acid (J. Am. Chem. Soc. 1962, 84, 284–292) was accomplished by Gilbert Stork. In the course of this synthesis, Stork discovered his famous enamine reaction.
(a) Write detailed mechanisms for the reactions from 6 to 8 below.
(b) Write detailed mechanisms for all of the reactions from 9 to 11 in Stork’s synthesis of dehydroabietic acid. Note that 11 contains a dithioacetal, which forms similarly to acetals you have already studied (Chapter 16).
(Fleming, I., Selected
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
ORGANIC CHEM.I-W/STD.GDE.+...>CUSTOM<
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Organic Chemistry (9th Edition)
Elementary Principles of Chemical Processes, Binder Ready Version
Chemistry & Chemical Reactivity
Chemistry: The Molecular Nature of Matter
Principles of Chemistry: A Molecular Approach (3rd Edition)
- Predict the coupling products of organometallic substitutions, and use them in syntheses.arrow_forwardIn an aqueous solution containing sodium bicarbonate, aniline reacts quickly withbromine to give 2,4,6-tribromoaniline. Nitration of aniline requires very strong conditions,however, and the yields (mostly m-nitroaniline) are poor.(a) What conditions are used for nitration, and what form of aniline is present under theseconditions?arrow_forwardDraw the chemical structure of a 1,2,3-triazole and showv how it can be synthesized from (b) basic starting materials. Comment on the acidity and basicity of triazoles. Comment on their reactivity towards electrophilic aromatic substitution relative to pyridines and pyrroles.arrow_forward
- Provide the reagents to carry out the following reactionarrow_forwardA common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of lithium metal. The Birch reduction usually reduces the aromatic ring, but in this case it eliminates the hydroxy group of ephedrine to give methamphetamine. Propose a mechanism, similar to that for the Birch reduction, to explain this unusual course of the reaction.arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

