
(a)
Interpretation:
The galvanic cell needs to be drawn and the flow of electron from right to left in the wire needs to be shown.
Concept Introduction: A galvanic cell is an
(a)
Explanation of Solution
A systematic galvanic cell is represented as follows:
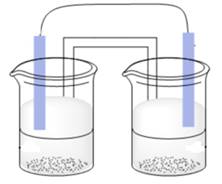
Here, according to question flow of electron takes place from right to left thus, arrows can be drawn with head directing towards left and tail towards the right.
Therefore, the flow of electrons is represented as follows:
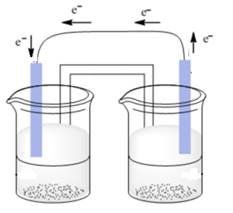
(b)
Interpretation:The anode and cathode need to be labelled.
Concept Introduction: A galvanic cell is an electrochemical cell where chemical energy is converted into electrical energy. It consists of two compartments where two electrodes are present. These two compartments are connected via salt-bridge.
(b)
Explanation of Solution
In a galvanic cell, cathode is an electrode where reduction takes place and electrons are added, but anode is an electrode where oxidation takes place and electrons are removed or released.
Thus, electrode on left hand side is cathode as electrons are flowing into that electrode and electrode on right hand side is anode as electrons are flowing from that electrode.
The cathode and anode are represented as follows:
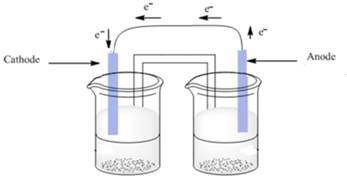
(c)
Interpretation: This is to be shown where oxidation and reduction takes place.
Concept Introduction: A galvanic cell is an electrochemical cell where chemical energy is converted into electrical energy. It consists of two compartments where two electrodes are present. These two compartments are connected via salt-bridge.
(c)
Explanation of Solution
In agalvanic cell, cathode is an electrode where reduction takes place. Here, electrons are added thus, the reaction is represented as follows:
The anode is an electrode where oxidation takes place. Here, electrons are removed or released thus, the reaction is represented as follows:
Thus, reduction and oxidation are labelled in the galvanic cell as follows:
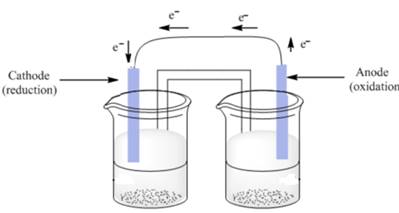
(d)
Interpretation: The change in the cell if salt bridge is removed needs to be explained.
Concept Introduction: A galvanic cell is an electrochemical cell where chemical energy is converted into electrical energy. It consists of two compartments where two electrodes are present. These two compartments are connected via salt-bridge.
(d)
Explanation of Solution
In the galvanic cell, the U-shaped tube joining two compartments of the cell is salt bridge.
It allows the movement of ions from one electrode solution to other, but it also controls the excessive mixing of the solutions. If salt bridge is removed, accumulation of charge takes place in both the compartments and no flow of electrons and current takes place. It is required so that the net charge in each of the compartment will be zero.
Chapter 18 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





