
Concept explainers
a)
Interpretation:
A
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
The change in free-energy represents the maximum amount of useful work that can be obtained in a reaction:
Relation between
Relation between
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
a)
Explanation of Solution
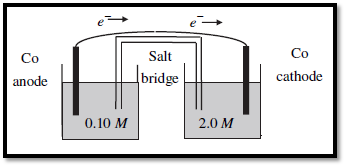
Figure.1
A galvanic concentration cell, each compartment consists of Co electrode in
Nernst equation of the concentration cell and Substitute known constant values of R, T and F into Nernst equation becomes as follows,
The number of electrons transferred in the given
The emf of the given galvanic cell reaction is
b)
Interpretation:
The concentrations in the compartments when
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Thermodynamics of redox reactions:
The change in free-energy represents the maximum amount of useful work that can be obtained in a reaction:
Relation between
Relation between
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
b)
Explanation of Solution
As a concentration cell runs, the concentration of the two solutions approaches each other. Let concentration of the dilute solution equal
The number of electrons transferred in the given redox reaction is TWO (n=2) and
Solve for x as follows,
At anode compartment:
At cathode compartment:
Want to see more full solutions like this?
Chapter 19 Solutions
Package: General Chemistry with Connect 2-year Access Card
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





