
Concept explainers
a)
Interpretation:
Half reactions; the anode and cathode have to be labelled.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
The change in free-energy represents the maximum amount of useful work that can be obtained in a reaction:
Relation between
Relation between
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
a)
Explanation of Solution
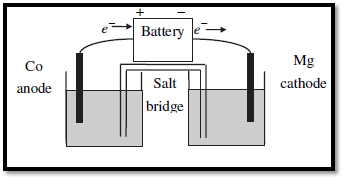
Figure.1
For the given redox reactions,
In the galvanic cell, Oxidation occurs at anode and reduction occurs at cathode.
Therefore,
Anode (Oxidation):
Cathode (Reduction):
b)
Interpretation:
The minimum voltage needed to drive the reaction has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
b)
Explanation of Solution
The emf values for the two given half-reactions are,
Anode (Oxidation):
Cathode (Reduction):
Calculated standard emf for galvanic cell as follows,
The minimum voltage needed to drive the reaction is
c)
Interpretation:
The emf of a given galvanic cell has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
c)
Explanation of Solution
Given: Current= 10.0 A; Time,
Convert Current into coulomb:
As known,
Convert number of Coulombs into mole of electrons:
Convert mole of electrons into number of moles:
1 mole of Cobalt
‘X’of Cobalt =
Therefore, no.of moles of Cobalt is
Assuming solution volumes of 1.00L, the concentration of Co2+ in solution after 2hours is 2.373 M, and the concentration of Mg2+ in solution after 2 hours is 1.627 M. we use the Nernst equation to solve for
Calculation of non-standard emf value using Nernst equation:
The reaction quotient for the given reaction is,
The concentration of pure solids and pure liquids do not appear in the expression for Q.
Hence, the reaction quotient becomes,
Substitute known constant values of R, T and F into Nernst equation becomes as follows,
The number of electrons transferred in the given redox reaction is TWO (n=2) and
The emf of the given cell reaction is
Want to see more full solutions like this?
Chapter 19 Solutions
Package: General Chemistry with Connect 2-year Access Card
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





