
(a)
The P - Vdiagram of the cycle, work done by the gas, heat absorbed by the gas and change in internal energy of the gas.
(a)
Answer to Problem 31P
The work done by the gas in each step are
Explanation of Solution
Given:
The initial pressure of the gas is
The initial volume of the gas is
The pressure at state 2 and 3 is
The volume at state 3 and 4 is
The pressure final pressure of the gas is
Formula used:
The expression for the specific heat ratio is given as,
Here,
The expression for heat absorbed by the gas in stage 1-2 is given as,
The expression for heat absorbed by the gas in stage 2-3 is given as,
The expression for heat absorbed by the gas in stage 3-4 is given as,
The expression for heat absorbed by the gas in stage 4-1 is given as,
The expression for the work done in stage 2-3 is given as,
The expression for the work done in stage 4-1 is given as,
The expression for change in internal energy in stage 1-2 is given as,
The expression for change in internal energy in stage 2-3 is given as,
The expression for change in internal energy in stage 3-4 is given as,
The expression for change in internal energy in stage 4-1 is given as,
Calculation:
The P-V diagram for the cycle can be given as,
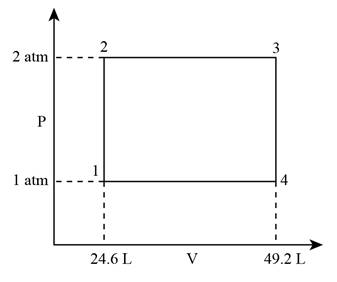
Figure 1
For monoatomic gas specific heat at constant volume and constant pressure can be given as,
The specific heat ratio can be calculated as,
The heat absorbed by the gas in stage 1-2 can be calculated as,
The expression for heat absorbed by the gas in stage 2-3 is given as,
The expression for heat absorbed by the gas in stage 3-4 is given as,
The expression for heat absorbed by the gas in stage 4-1 is given as,
The work done in stage 1-2 is zero because it is constant volume process.
The work done in stage 2-3 can be calculated as,
The work done in stage 3-4 is zero because it is constant volume process.
The work done in stage 4-1 can be calculated as,
The expression for change in internal energy in stage 1-2 is given as,
The expression for change in internal energy in stage 2-3 is given as,
The expression for change in internal energy in stage 3-4 is given as,
The expression for change in internal energy in stage 4-1 is given as,
Conclusion:
Therefore,the work done by the gas in each step are
(b)
The efficiency of the cycle.
(b)
Answer to Problem 31P
The efficiency of the cycle is
Explanation of Solution
Formula used:
The expression for total heat supplied to the cycle is given as,
The expression for total work done in the cycle is given as,
The expression for efficiency of the cycle is given as,
Here,
Calculation:
The total heat supplied to the cycle can be calculated as,
The total work done in the cycle can be calculated as,
The efficiency of the cycle can be calculated as,
Conclusion:
Therefore, the efficiency of the cycle is
Want to see more full solutions like this?
Chapter 19 Solutions
Physics For Scientists And Engineers
- If a gas is compressed isothermally, which of the following statements is true? (a) Energy is transferred into the gas by heat. (b) No work is done on the gas. (c) The temperature of the gas increases, (d) The internal energy of the gas remains constant, (e) None of those statements is true.arrow_forwardWhen 400 J of heat are slowly added to 10 mol of an ideal monatomic gas, its temperature rises by 10 . What is the work done on the gas?arrow_forwardA Carnot engine employs 1.5 mol of nitrogen gas as a working substance, which is considered as an ideal diatomic gas with =7.5 at the working temperatures of the engine. The Carnot cycle goes in the cycle ABCDA with AB being an isothermal expansion. The volume at points A and C of the cycle are 5.0103 m3 and 0.15 L, respectively. The engine operates between two thermal baths of temperature 500 K 300 K. (a) Find the values of volume at B and D. (b) How much heat is absorbed by the gas in the AB isothermal expansion? (c) How much work is done by the gas in the AB isothermal expansion? (d) How much heat is given up by the gas in the CD isothermal expansion? (e) How much work is done by the gas in the CD isothermal compression? (f) How much work is done by the gas in the BC adiabatic expansion? (g) How much work is done by the gas in the DA adiabatic compression? (h) Find the value of efficiency of the engine based on the net and heat input. Compare this value to the efficiency of a Carnot engine based on the temperatures of the baths.arrow_forward
- An amount of n moles of a monatomic ideal gas in a conducting container with a movable piston is placed in a large thermal heat bath at temperature T1 and the gas is allowed to come to equilibrium. After the equilibrium is leached, the pressure on the piston is lowered so that the gas expands at constant temperature. The process is continued quasi-statically until the final pressure is 4/3 of the initial pressure p1 . (a) Find the change in the internal energy of the gas. (b) Find the work done by the gas. (c) Find the heat exchanged by the gas, and indicate, whether the gas takes in or gives up heat.arrow_forwardTwo moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four pi-cresses in the pV plane. (b) Find the total work done by the gas.arrow_forwardWhich of the following is true for the entropy change of a system that undergoes a reversible, adiabatic process? (a) S 0 (b) S = 0 (c) S 0arrow_forward
- A Carnot engine working between two heat baths of temperatures 600 K and 273 K completes each cycle in 5 sec. In each cycle, the engine absorbs 10 kJ of heat. Find the power of the engine.arrow_forwardAn ideal gas with specific heat ratio confined to a cylinder is put through a closed cycle. Initially, the gas is at Pi, Vi, and Ti. First, its pressure is tripled under constant volume. It then expands adiabatically to its original pressure and finally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the volume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expansion and (d) the temperature at the end of the cycle. (e) What was the net work done on the gas for this cycle?arrow_forwardTwo moles of nitrogen gas, with =7/5 for ideal diatomic gases, occupies a volume of 102 m3 in an insulated cylinder at temperature 300 K. The gas is adiabatically and reversibly compressed to a volume of 5 L. The piston of the cylinder is locked in its place, and the insulation around the cylinder is removed. The heat-conducting cylinder is then placed in a 300-K bath. Heat from the compressed gas leaves the gas, and the temperature of the gas becomes 300 K again. The gas is then slowly expanded at the fixed temperature 300 K until the volume of the gas becomes 102 m3, thus making a complete cycle for the gas. For the entire cycle, calculate (a) the work done by the gas, (b) the heat into or out of the gas, (c) the change in the internal energy of the gas, and (d) the change in entropy of the gas.arrow_forward
- A thermodynamic system undergoes a process in which its internal energy decreases by 500 J. Over the same time interval, 220 J of work is done on the system. Find the energy transferred from it by heat.arrow_forwardThere is no change in the internal of an ideal gas undergoing an isothermal process since the internal energy depends only on the temperature. Is it therefore correct to say that an isothermal process is the same as an adiabatic process for an ideal gas? Explain your answer. `arrow_forwardA 1.00-mol sample of an ideal gas ( = 1.40) is carried through the Carnot cycle described in Figure 22.11. At point A, the pressure is 25.0 atm and the temperature is 600 K. At point C, the pressure is 1.00 atm and the temperature is 400 K. (a) Determine the pressures and volumes at points A, B, C, and D. (b) Calculate the net work done per cycle.arrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





