
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Biochemistry: Concepts and Connections
1st Edition
ISBN: 9780133882797
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 15P
The anno acid arginine ionizes according to the following scheme:
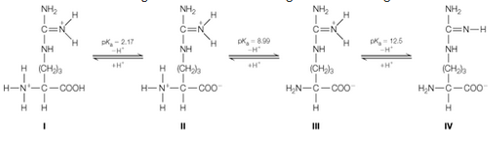
a. Calculate the isoelectric point of arginine. You can neglect contributions from form l. Why?
b. Calculate the average charge on arginine when pH = 9.20. (Hint: Find the average charge for each ionizable group and sum these together)
c. Is the value of average charge you calculated in part b reasonable, given the pl you calculated part a? Explain your answer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The amino acid arginine ionizes according to the following scheme: (a) Calculate the isoelectric point of arginine. You can neglect contributionsfrom form I. Why?(b) Calculate the average charge on arginine when pH = 9.20.(c) Is the value of average charge you calculated in part b reasonable, giventhe pI you calculated in part a? Explain your answer.
The amino acid arginine ionizes according to the following scheme: (a) Calculate the isoelectric point of arginine. You can neglect contributions from form I. Why? (b) Calculate the average charge on arginine when pH = 9.20. (c) Is the value of average charge you calculated in part b reasonable, given the pI you calculated in part a? Explain your answer
For the following pentapeptides: Ser-Glu-Gly-His-Ala and Gly-His-Ala-Glu-Ser
A. Compute their isoelectric pH (pI). Show full solution. Use standard pKa values.
B. Do these peptides with the same amino acid composition have different net charges at pH 7.0? Explain briefly.
C. Would you expect the titration curves of the two peptides to differ? Why or Why not?
Chapter 2 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Biochemistry: Concepts and Connections
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - Prob. 20PCh. 2 - Prob. 21P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- The ionization of p-nitrophenol is shown below (pKa = 7.0): a. Identify the weak acid and conjugate base. b. At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol? c. If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms? d. Ionized p-nitrophenol has a yellow color, while the un-ionized form is colorless. The yellow color can be measured using a spectrophotometer at 400nm. In order to determine the total amount of p-nitrophenol in a solution, would you perform the spectrophotometer reading at an acidic or basic pH? Clearly explain why? e. A solution of p-nitrophenol at pH 7.95 was found to have an A400 of 0.255 . What is the total concentration (in µM) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 18,500 M-1cm-1 and the pKa is 7.arrow_forwardIf crystals of deoxy Hb are exposed to air, they break. What does this imply about the 4o structure of deoxy Hb and oxy Hb and predict which subunits junctions are most affected in the change from deoxyHb to oxyHb?arrow_forward(a) What is the net charge at neutral pH of a tripeptide containing only alanine? (b) How does the total number of negative and positive charges change following hydrolysis of the tripeptide?arrow_forward
- Draw the peptide ASK(ala-ser-lysine) with proper stereochemistry? what is its pI(isoelectric point)and why the structure is planar? Use the following table to look at pka's of the amino acids and solve this question.arrow_forwardConsider a peptide with the sequence Ala-Glu-Arg-Leu. Assume the ionizable groups have the pKa values listed in Table 2.1 of your text. (a) Draw the predominant ionic form of the peptide at pH 7.4. (b) Determine the net charge of the predominant form of the peptide at pH 7.4. (c) Calculate the pI of the peptide.arrow_forwardGiven the titration curve of the hypothetical polyprotic acid X at 0.100 M concentration (pKa1=4.0, pKa2=8.0, pKa3=12.0) titrated with 0.600 M NaOH, identify the pH at point C, H, E, and M.arrow_forward
- The β chains of HbA and HbS were treated with trypsin, and the sequence of the N-terminal tryptic peptides are as given below. Do these peptides separate from each other in an electric field if the pH is 7.0? Explain in detail the reasoning behind your answer and include your calculations for the charge of each peptide in your answer.arrow_forwardDraw Ramachandran plot for: a) regular secondary structure with Φ = 60-65 and Ψ = 60-80 b) regular secondary structure with Φ = -170 and Ψ = 170 c) intrinsically disordered proteins d) explain why these Ramachsndran plots will be different, and what secondary structures are described in A and in Barrow_forwardThe melting curve for the polyribonucleotide poly(A) is shown below. (a) Explain why absorbance increases with increasing temperature. (b) Why does the shape of the curve differ from the one shown in Fig. ?arrow_forward
- Let’s consider histidine as a free amino acid in aqueous solution. a) Draw the most likely structure of histidine under biochemical standard state conditions. b) Given that free histidine has the following three pKa values, assign each to its corresponding acidic hydrogen or conjugate base in your structure from part a). pKa1 = 1.7; pKa2 = 6.2; and pKa3 = 9.1 c) For each pKa, give the corresponding expression for the equilibrium constant. It helps to write out the chemical equation for each. d) Create a speciation diagram for histidine by plotting Xi vs pH from pH = 4 to pH = 8 where Xi is the mole fraction of the two histidine species involved in the K2 equilibrium in part c).arrow_forwardyou have isolated the following peptide: His-Ser-Arg- Ala-Glu- Leu- Pro- Gly Calculate the approximate charge of the peptide at Ph 1, 3, 5, 8, 11, 14 AND what is the PI of this peptide?arrow_forwardClassify the fatty acid with the following structural formula in the ways indicated. a. What is the type designation (SFA, MUFA, or PUFA) for this fatty acid? b. On the basis of carbon chain length and degree of unsaturation, what is the numerical shorthand designation for this fatty acid? c. To which "omega" family of fatty acids does this belong? d. What is the "delta" designation for the carbon chain double-bond locations for this fatty acid? Note: There are 2 items in the photo.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license