
Concept explainers
Interpretation:
Molecular geometry and electron geometry about each non-hydrogen atom in the given molecule is to be predicted using VSEPR theory.
Concept introduction:
Electron geometry and molecular geometry of molecules are determined by using Valence shell electron pair repulsion (VSEPR) theory. According to VSEPR theory, electron geometry describes the orientation of the electron groups about a particular atom and molecular geometry describes the arrangement of atoms about a particular atom.
The number of electron pairs describes the electron and molecular geometry. If all the electron pairs are bonds, then the molecular geometry is the same as the electron geometry. Electron geometry is different from molecular geometry if some electron groups are present as lone pairs. The bond angle depends on the electron geometry around the atom.
Electron geometry and molecular geometry from the number of electron pairs and bond angle according to VSEPR theory are as follows:
| Number of Electron Groups |
Number of Bonds |
Number of Lone Pairs |
Bond Angle (o) |
Electron Geometry | Molecular Geometry |
| 2 | 2 | 0 | 180 | Linear | Linear |
| 3 | 3 | 0 | 120 | Trigonal planar | Trigonal planer |
| 3 | 2 | 1 | 120 | Trigonal planar | Bent |
| 4 | 4 | 0 | 109.5 | Tetrahedral | Tetrahedral |
| 4 | 4 | 0 | 180 | Linear | Linear |
| 4 | 2 | 2 | 109.5 | Tetrahedral | Bent |
Answer to Problem 2.2P
According to VSEPR theory, the electron and molecular geometry about each of the non-hydrogen atom in the structure is as follows:
Oxygen = Electron geometry is tetrahedral while molecular geometry is bent.
C1 carbon atom = Electron geometry is tetrahedral while molecular geometry is also tetrahedral.
C2 and C3 carbon atoms = Electron geometry is linear while molecular geometry is also linear.
Explanation of Solution
The given structure for
The structure showing all the atoms and lone pairs is:
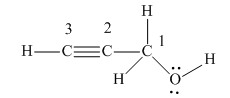
There are four non-hydrogen atoms in the above structure. They are numbered from 1 to 4.
There are four groups of electrons around the oxygen atom: two lone pairs of electrons and two single bonds. According to VSEPR theory, its electron geometry is tetrahedral, and its molecular geometry is bent.
There are four groups of electrons around the C1 carbon: four single bonds and no lone pairs of electrons. According to VSEPR theory, its electron geometry is tetrahedral, and its molecular geometry is also tetrahedral.
There are two groups of electrons around the C2 carbon: one triple bond, and one single bond, and no lone pairs of electrons. According to VSEPR theory, its electron geometry is linear, and its molecular geometry is also linear.
There are two groups of electrons around the C3 carbon: one triple bond, one single bond, and no lone pairs of electrons. According to VSEPR theory, its electron geometry is linear, and its molecular geometry is also linear.
The electron geometry and molecular geometry about each non-hydrogen atom in the given molecule is predicted on the basis of VSEPR chart.
Want to see more full solutions like this?
Chapter 2 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Incorrect. This bond is made of an sp3 orbital from a carbon atom to an sp orbital on the other carbon, containing the triple bond. The correct orbital overlap is sp3-sp. What type(s) of orbital overlap is(are) indicated on the following structure:arrow_forwardDraw example of meso compound with the formula C8H18arrow_forwardall one problem, fill in the blanks, draw the arrowsarrow_forward
- Can you check if I drawn a correct MO energy diagram for ethylene. And is the HOMO and LUMO correct?arrow_forwardAnswer the following question about acetonitrile (CH3C≡N:). Question: Label all bonds as σ or π.arrow_forwarddraw 2 models of Bromochloromethanol isomer Which one is r and a s? Determine the result of odd # and even # interchangesarrow_forward
- Select if the following statements are true or false. Group of answer choices In VB theory, all the electrons in the structure are delocalized. The number of hybridized orbitals always equals the number of atomic orbitals on the central atom. VB theory accurately predicts bond strength, angle and length. According to VB theory, the more overlap of orbitals, the longer the bond length.arrow_forwardPlease solve, with explanation. Need only 4.18 determine whether the following compounds are constitutional isomersarrow_forwardI’m not sure how to draw this where there would only be one chiral carbon AND be branchedarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
