
Concept explainers
(a)
Interpretation:
The electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule is to be identified.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron and molecular geometry about the charged atom in the given molecular ion is trigonal planar and the bond angle is
Explanation of Solution
The given molecular ion is:
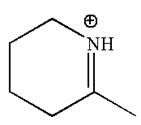
In this molecular ion, the charged atom is nitrogen. Nitrogen has two single bonds and one double bond thus, there are three electron groups around the nitrogen atom. According to the VSEPR chart, the electron geometry about nitrogen is trigonal planar and as there are no lone pairs around it, the molecular geometry is also trigonal planar. The bond angle in the orientation of three electron groups is
The electron geometry, molecular geometry and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
(b)
Interpretation:
The electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule is to be identified.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron geometry about the charged atom in the given molecular ion is trigonal planar, molecular geometry is bent and the bond angle is
Explanation of Solution
The given molecular ion is:
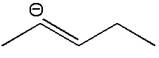
In this molecular ion, the charged atom is carbon; the negative charge indicates the lone pairs of electrons. The charged carbon atom has one single bond and one double bond and one lone pair thus there are three electron groups around this charged carbon atom. According to the VSEPR chart, the molecular geometry is bent, since there are two bonds around the charged carbon atom. And the electron geometry about charged carbon is trigonal planar as there is one lone pairs of electrons along with two bonds. The bond angle in the orientation of three electron groups is
The electron geometry, molecular geometry and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
(c)
Interpretation:
The electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule is to be identified.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron geometry about the charged atom in the given molecular ion is trigonal planar, molecular geometry is bent and the bond angle is
Explanation of Solution
The given molecular ion is:
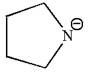
In this molecular ion, the charged atom is nitrogen; the negative charge indicates the lone pairs of electrons. The charged nitrogen atom has two single bonds and one lone pair of electrons, thus there are three electron groups. According to the VSEPR chart, the electron geometry about charged nitrogen should be trigonal planar and as there is one lone pair around it, the molecular geometry is bent. The bond angle in the orientation of three electron groups is
The electron geometry, molecular geometry, and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
(d)
Interpretation:
It is to be identified the electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron and molecular geometry about the charged atom in the given molecular ion is tetrahedral and the bond angle is
Explanation of Solution
The given molecular ion is:
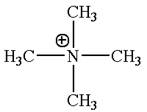
In this molecular ion, the charged atom is nitrogen. The charged nitrogen atom has four single bonds and no lone pair thus there are four electron groups. According to the VSEPR chart the electron geometry about charged nitrogen should be tetrahedral and as there is no lone pair around it, the molecular geometry as same as the electron geometry, that is tetrahedral. The bond angle in the orientation of four electron groups is
The electron geometry, molecular geometry and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
(e)
Interpretation:
It is to be identified the electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron and molecular geometry about the charged atom in the given molecular ion is trigonal planar and the bond angle is
Explanation of Solution
The given molecular ion is:
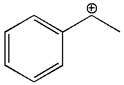
In this molecular ion, the charged atom is carbon. The carbon atom bearing the positive charge has three bonds, two with carbons and one with hydrogen. This carbon has an incomplete octet and thus it has no lone pairs of electrons on it. Thus there are three electron groups around this carbon atom. According to the VSEPR chart, the electron geometry about charged carbon is trigonal planar and as there is no lone pair around it, the molecular geometry is also trigonal planar. The bond angle in the orientation of four electron groups is
The electron geometry, molecular geometry and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
(f)
Interpretation:
The electron geometry, molecular geometry, and bond angle about the charged atom in the given molecule is to be identified.
Concept introduction:
The VSEPR chart depicts the electron and molecular geometry on the basis of numbers of electron groups. The Electron geometry about an atom describes the orientation of a group of electrons around it. A lone pair of electrons, a single bond, a double bond, a triple bond each is considered as one group of electrons. Molecular geometry describes the arrangement of surrounding atoms about a particular atom. Molecular geometry is governed by electron geometry of an atom. If all the electron groups around an atom are bonds, then the molecular geometry will be the same as electron geometry. The electron and molecular geometry about a particular atom will be different when one or more of the electron group is lone pair. The bond angle is the angle between two bonds originating from the same atom in a covalent molecule or ion. The bond angle depends on the molecular geometry around the atom.
Answer to Problem 2.34P
The electron and molecular geometry about the charged atom in the given molecular ion is linear and the bond angle is
Explanation of Solution
The given molecular ion is:
![]()
In this molecular ion, the charged atom is oxygen. The charged oxygen atom has one triple bond and one lone pair thus there are two electron groups. According to the VSEPR chart, the two electron group tends to have linear electron and molecular geometry. The bond angle in the orientation of tow electron groups is
The electron geometry, molecular geometry and the bond angle about charged atom in the given molecule is predicted on the basis of VSEPR chart.
Want to see more full solutions like this?
Chapter 2 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Methylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) Give values for the three bond angles indicated. (b) Indicate the most polar bond in the molecule. (c) Circle the shortest carbon-oxygen bond. (d) Circle the shortest carbon-carbon bond.arrow_forwardA student who missed this class needs to know how to predict the bond angles and shape of amolecule from looking at its bond-line representation. Write a concise but complete explanationfor this student.arrow_forwardWhat is the most polar bond in the molecule?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





