
Concept explainers
(a)
Interpretation:
To accurately depict the given ball-stick model, the dash-wedge notations in the given line structure are to be added.
Concept introduction:
The dash-wedge notations are used to represent the three-dimensional arrangement of atoms or groups of molecules in plane of paper. There are three types of lines used for such arrangements, straight line, dash line, and wedge line. The straight line represents the bond is in the plane of the paper. The dash line represents the bond pointing away from the observer, that is, below the plane of paper. The wedge line represents the bond pointing towards the observer that is above the plane of paper.
Answer to Problem 2.36P
The dash-wedge notations in the given line structure is:
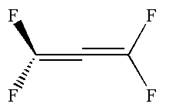
Explanation of Solution
The given ball-stick model and the line structure are:
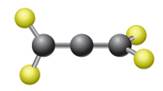
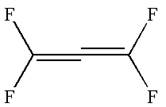
In the given ball-stick model, the yellow balls represent fluorine atoms and black balls are carbon atoms. The fluorine atoms on the left side appear in the plane of the paper. Thus, these fluorine atoms will be represented by straight lines. The two fluorine atoms on the right side are arranged such that, one fluorine atom is above the plane of paper and other is below. The one which is above the plane will be represented by a wedge bond while the one which is below the plane of the paper will be represented by a dash line.
Hence dash-wedge notation for the given molecules is shown below:
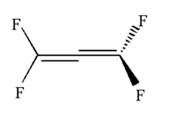
The dash-wedge notation in the given line structure is added on the basis of three dimensional arrangement given in ball-stick model.
(b)
Interpretation:
To accurately depict the given ball-stick model, the dash-wedge notations in the given line structure are to be added.
Concept introduction:
The dash-wedge notations are used while representing the three dimensional arrangement of atoms or groups of molecules on the plane of paper. There are three types of lines used for such arrangements, straight line, dash line, and wedge line. The straight line represents the bond that is in the plane of the paper. The dash line represents the bond pointing away from the observer that is below the plane of paper. The wedge line represents the bond pointing towards the observer that is above the plane of paper.
Answer to Problem 2.36P
The dash-wedge notations in the given line structure is:
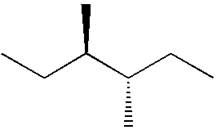
Explanation of Solution
The given ball-stick model and its line structure are:
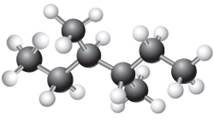
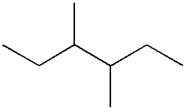
In the given ball-stick model, the white balls represent hydrogen atoms while black balls are carbon atoms. The longest continuous chain is of six carbon atoms having two methyl substituents at
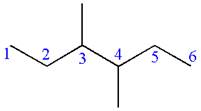
If the chain is numbered from left to right, the methyl group at
Hence, the dash-wedge notation for the given molecules is shown below:
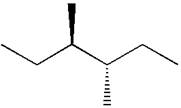
The dash-wedge notation in the given line structure is added on the basis of three dimensional arrangement given in ball-stick model.
(c)
Interpretation:
To accurately depict the given ball-stick model, the dash-wedge notations in the given line structure are to be added.
Concept introduction:
The dash-wedge notations are used while representing the three dimensional arrangement of atoms or groups of molecules on the plane of paper. There are three types of lines used for such arrangements, straight line, dash line, and wedge line. The straight line represents the bond is in the plane of paper. The dash line represents the bond pointing away from the observer that is below the plane of paper. The wedge line represents the bond pointing towards the observer that is above the plane of paper.
Answer to Problem 2.36P
The dash-wedge notations in the given line structure is:
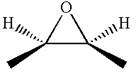
Explanation of Solution
The given ball-stick model and its line structure are:
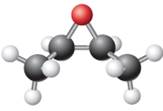
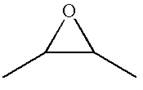
In the given ball-stick model the white balls represent hydrogen atoms, black balls represents carbon atoms and the red ball is an oxygen atom. The two carbon atoms that are directly bonded to the oxygen atom are in the plane of paper. Each of these carbon atoms has one methyl substituent and one hydrogen atom attached. Two methyl substituents points towards the observer, while the hydrogen atoms are pointing away from the observer. The bond towards the observer is considered as above the plane of the paper and is represented by wedge bond. The bond away from the observer is considered as below the plane of the paper and is represented by dash bond.
Hence dash-wedge notation for the given molecules is shown below:
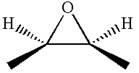
The dash-wedge notation in the given line structure is added on the basis of three dimensional arrangement given in ball-stick model.
(d)
Interpretation:
To accurately depict the given ball-stick model, the dash-wedge notations in the given line structure are to be added.
Concept introduction:
The dash-wedge notations are used while representing the three dimensional arrangement of atoms or groups of molecules on the plane of paper. There are three types of lines used for such arrangements, straight line, dash line, and wedge line. The straight line represents the bond is in the plane of the paper. The dash line represents the bond pointing away from the observer that is below the plane of paper. The wedge line represents the bond pointing towards the observer that is above the plane of paper.
Answer to Problem 2.36P
The dash-wedge notations in the given line structure is:
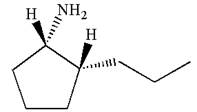
Explanation of Solution
The given ball-stick model and its line structure are:
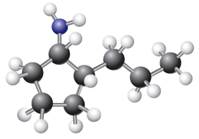
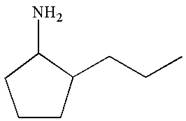
In the given ball-stick model the white balls represent hydrogen atoms, black balls are carbon atoms, and the blue ball is a nitrogen atom. The cyclopentane ring is on the plane of paper having two substituents. The
The
The second substituent is propyl group which points away from the observer and the hydrogen atoms bonded to the same carbon having propyl group is pointed towards observer.
The bond towards the observer is considered as above the plane of the paper and is represented by a wedge line. The bond away from the observer is considered as below the plane of the paper and is represented by dash line
Hence dash-wedge notation for the given molecules is shown below:
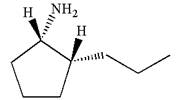
The dash-wedge notation in the given line structure is added on the basis of three dimensional arrangement given in ball-stick model.
Want to see more full solutions like this?
Chapter 2 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





