
Interpretation:
Partial columns has to be filled in the table given in problem statement.
Concept Introduction:
Each and every element present in the Periodic table has a unique name. Some of the elements are named considering their
Chemical names are represented as atomic symbols. In the symbols, the mass number and atomic number are shown. The complete
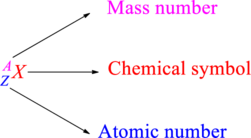
Atomic number is the total number of protons present in the atom of an element. Mass number is the total number of protons and neutrons present in nucleus of an atom.
Explanation of Solution
For entry 1:
Atomic number is given as 11, number of electrons is given as 10, and number of neutrons is given as 12. Total number of protons present in the nucleus is the atomic number. Therefore, the number of protons present is 11.
Mass number is the sum of number of protons and number of neutrons. This can be calculated as shown below.
Charge can be calculated by finding the difference between the number of protons and electrons as shown below.
Atomic number is given as 11. The element with atomic number 11 is found to be sodium which has a symbol as
For entry 2:
Symbol is given as
Mass number is the sum of number of protons and number of neutrons. From the mass number given as 40 in the symbol, the number of neutrons can be calculated as shown below.
Charge is given as
For entry 3:
Number of protons is given as 35. This means that the atomic number of the species is 35. The element with atomic number 35 is found to be bromine with the symbol of
Mass number is the sum of number of protons and number of neutrons. From the mass number given as 81, the number of neutrons can be calculated as shown below.
Charge is given as
For entry 4:
Number of protons is given as 52. This means that the atomic number of the species is 52. The element with atomic number 52 is found to be tellurium with the symbol of
Mass number is the sum of number of protons and number of neutrons. This can be calculated as shown below.
Charge is given as
Complete table can be given as shown below.
| Symbol | ||||
| Atomic number | ||||
| Mass number | ||||
| Charge | ||||
| Number of protons | ||||
| Number of electrons | ||||
| Number of neutrons |
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry: Principles and Practice
- Complete the following table by filling in the blanks in each row. The first row has been completed as an example.arrow_forwardAssume silicon has three major isotopes in nature as shown in the table below. Fill in the missing information. Isotope Mass (u) Abundance 28Si 27.98 _____ 29Si ____ 4.70% 30Si 29.97 3.09%arrow_forwardTwo samples of different compounds of nitrogen and oxygen have the following compositions. Show that the compounds follow the law of multiple proportions. What is the ratio of oxygen in the two compounds for a fixed amount of nitrogen? Amount N Amount O Compound A 1.206 g 2.755 g Compound B 1.651 g 4.714 garrow_forward
- White phosphorus is available in sticks, which have a waxy appearance. This is a molecular substance, P4. When this solid is vaporized, it first forms P4 molecules; but at high temperature, P2 molecules are formed. How do the molecules of white phosphorus and those of the hot vapor differ? How are the molecules alike?arrow_forwardAnswer the questions below, using LT (for is less than), GT (for is greater than), EQ (for is equal to), or MI (for more information required) in the blanks provided. (a) The mass (to three significant figures) of6.0221023atoms of Na 23.0 g. (b) Boron has two isotopes, B-10 (10.01 amu) and B-11 (11.01 amu). The abundance of B-10 the abundance of B-11. (c) If S-32 were assigned as the standard for expressing relative atomic masses and assigned an atomic mass of 10.00 amu, the atomic mass for H would be 1.00 amu. (d) When phosphine gas, PH3, is burned in oxygen, tetraphosphorus decaoxide and steam are formed. In the balanced equation (using smallest whole-number coefficients) for the reaction, the sum of the coefficients on the reactant side is 7. (e) The mass (in grams) of one mole of bromine molecules is 79.90.arrow_forwardTwo samples of different compounds of sulfur and oxygen have the following compositions. Show that the compounds follow the law of multiple proportions. What is the ratio of oxygen in the two compounds for a fixed amount of sulfur? Amount S Amount O Compound A l.210g 1.811 g Compound B 1.783 g 1.779 garrow_forward
- List some properties of a substance that would lead you to believe it consists of ions. How do these properties differ from those of nonionic compounds?arrow_forwardFill in the following table:arrow_forwardGive three examples of gaseous elements that exist as diatomic molecules. Give three examples of gaseous elements that exist as monatomic species.arrow_forward
- The normal form of the element sulfur is a brittle, yellow solid. This is a molecular substance, Sg. If this solid is vaporized, it first forms Sg molecules; but at high temperature, S2 molecules are formed. How do the molecules of the solid sulfur and of the hot vapor differ? How are the molecules alike?arrow_forwardWrite the chemical formula of each of the following: a The compound made up of a crystal with two particles coming from chromium atoms for every three particles coming from oxygen atoms. b The compound made up of a crystal with one particle coming from a barium atom for every two particles coming from chlorine atoms. c The compound made up of molecules with 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. d The compound made up of molecules with three hydrogen atoms, one phosphorus atom, and four oxygen atoms.arrow_forwardGiven that the periodic table is an organizational scheme for the elements, what might be some other logical ways in which to group the elements that would provide meaningful chemical information in a periodic table of your own devising?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





