
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.50SP
Label the reactants in these acid-base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
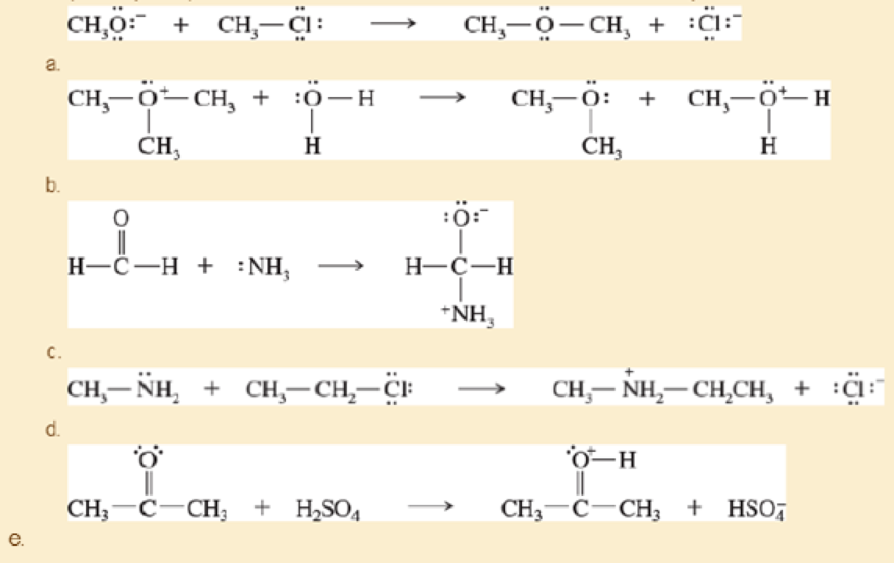
- f. (CH3)3CCl + AlCl3 → (CH3)3C+ + −AlCl4
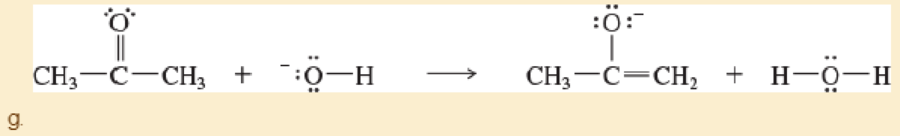
- h. CH2=CH2+BF3→BF–3—CH2—C+H2
- i. BF–3—CH2—C+H2+CH2=CH2→BF–3—CH2—CH2—CH2—CH+2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Disulfides are compounds that have S¬ S bonds, like peroxides have O¬ O bonds. Thiols are organic compounds that have the general formula R¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R¬ S¬ S¬ R. If you wanted to convert a disulfide to two thiols, should you add a reducing agent or oxidizing agent to the solution?
Fill in the left side of this equilibrium constant equation for the reaction of diethylmethylamine (C,H13N), a weak base, with water.
Ü - K,
Consider the molecules R-CH2OH and R-CH2CO2H where R is rest of the hydrocarbon molecule.
a) Which has the stronger conjugate base?
b) Explain why it is the stronger base. What about the structure helps us to understand why one is stronger than the other?
You may use drawings to help explain your answer.
Chapter 2 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 2.1A - Prob. 2.1PCh. 2.1B - The NF bond is more polar than the NH bond: but...Ch. 2.1B - For each of the following compounds 1. Draw the...Ch. 2.1B - Two isomers of 1,2-dichloroethene are known One...Ch. 2.2C - Prob. 2.5PCh. 2.2C - Prob. 2.6PCh. 2.3 - Prob. 2.7PCh. 2.4 - Calculate the pH of the following solutions a....Ch. 2.6A - Ammonia appears in Table 2-2 as both an acid and a...Ch. 2.7 - Write equations for the following acid-base...
Ch. 2.7 - Ethanol, methylamine. and acetic acid are all...Ch. 2.8 - Prob. 2.12PCh. 2.10 - Write equations for the following acid-base...Ch. 2.10 - Rank the following acids in decreasing order of...Ch. 2.11 - Prob. 2.15PCh. 2.11 - Prob. 2.16PCh. 2.11 - Consider each pair of bases and explain which one...Ch. 2.12 - Which is a stronger base ethoxide ion or acetate...Ch. 2.12 - Prob. 2.19PCh. 2.12 - Prob. 2.20PCh. 2.12 - Prob. 2.21PCh. 2.12 - Choose the more basic member of each pair of...Ch. 2.14 - Prob. 2.23PCh. 2.15D - Classify the following hydrocarbons and draw a...Ch. 2.16D - Prob. 2.25PCh. 2.17C - Draw a Lewis structure and classify each of the...Ch. 2.17C - Circle the functional groups in the following...Ch. 2 - The CN triple bond in acetonitrile has a dipole...Ch. 2 - Prob. 2.29SPCh. 2 - Sulfur dioxide has a dipole moment of 1.60 D....Ch. 2 - Which of the following pure compounds can form...Ch. 2 - Predict which member of each pair is more soluble...Ch. 2 - Prob. 2.33SPCh. 2 - Prob. 2.34SPCh. 2 - Predict which compound in each pair has the higher...Ch. 2 - All of the following compounds can react as acids...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - The Ka of phenylacetic acid is 5 2 105, and the...Ch. 2 - The following compound can become protonated on...Ch. 2 - The following compounds are listed in increasing...Ch. 2 - Prob. 2.42SPCh. 2 - Prob. 2.43SPCh. 2 - Compare the relative acidity of 1-molar aqueous...Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - The following compounds can all react as bases. a....Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - Prob. 2.48SPCh. 2 - Methyllithium (CH3Li) is often used as a base in...Ch. 2 - Label the reactants in these acid-base reactions...Ch. 2 - In each reaction, label the reactants as Lewis...Ch. 2 - Prob. 2.52SPCh. 2 - Each of these compounds can react as a nucleophile...Ch. 2 - Prob. 2.54SPCh. 2 - Give a definition and an example for each class of...Ch. 2 - Circle the functional groups in the following...Ch. 2 - Prob. 2.57SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the conjugate acid of H2C6H7O5 -1aq2? What is its conjugate base?arrow_forward2,4-Pentanedione is a considerably stronger acid than is acetone (Chapter 19). Write a structural formula for the conjugate base of each acid and account for the greater stability of the conjugate base from 2,4-pentanedione.arrow_forwardWrite these reactions as proton-transfer reactions. Label which reactant is the acid and which is the base, which product is the conjugate base of the original acid, and which is the conjugate acid of the original base. In addition, write Lewis structures for each reactant and product and use curved arrows to show the flow of electrons in reaction. Q.) CH3CH2OH + NH2+ --------> CH3CH2O2 + NH3arrow_forward
- Acetylsalicylic acid (aspirin) can be synthesized by combining salicylic acid and acetic acid through a condensation reaction. The −OH−OH group from phenol on the salicylic acid condenses with the carboxylic acid group of acetic acid forming acetylsalicylic acid. Draw the structure of acetylsalicylic acid.arrow_forwardC5H8O2 + NaH + HCl −→ C5H12O2 + NaClarrow_forwardTranslate the following reaction completely into English words using no symbols: 4NH3 + 5O2 --> 4NO + 6H2Oarrow_forward
- Write the second dissociation for both reactions. Dissociation reaction First dissociation CICH-COOH + H.О - Нз0* + CICH-COO- Hydrolysis First dissociation CICH2COO + H2O + OH- + CICH2COOHarrow_forwardConsider the following reactions. 2CH4 (g) ⇌ C2H6 (g) + H2 (g) Kc = 9.5x10–13 CH4 (g) + H2O (g) ⇌ CH3OH (g) + H2 (g) Kc = 2.8x10–21 Calculate the equilibrium constant, Kc value for the following reaction. 2CH3OH (g) + H2 (g) ⇌ C2H6(g) + 2H2O (g)arrow_forwardMg(OH)2 + H3PO4→ Mg3(PO«)2 + H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY