
Concept explainers
QUANTITATIVE Bond Energies. A single covalent bond has a bond energy of approximately 90 kcal/mol, and a typical hydrogen bond has a bond energy of about 5 kcal/mol. Although weak, hydrogen bonds can be a major structural force when present in large numbers as in DNA. In double-stranded DNA, each AT base pair is held together by two hydrogen bonds, and each GC base pair is held together by three hydrogen bonds.
(a) What is the total bond energy in a propane molecule (see Figure 2-4)? The C—C bond energy is 83 kcal/mol, and the C—H bond energy is 99 kcal/mol.
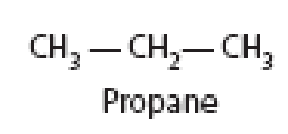
(b) In a short 15 base-pair molecule of DNA having 60% GC pairs and 40% AT pairs, what is the total bond energy of all the hydrogen bonds? How does this compare to the bond energy of a carbon-carbon bond?
(c) In a typical gene consisting of 1000 base pairs with the same relative GC versus AT content, what is the total bond energy of all the hydrogen bonds? How does this compare to the bond energy of a carbon-carbon bond?
Trending nowThis is a popular solution!

Chapter 2 Solutions
Becker's World of the Cell (9th Edition)
- In α-helices - what is the length of the hydrogen bonds between: TYR41H and GLU37O ALA39H and MET35O GLU38H and ILE34O THR36H and ARG32O. ARG32H and TYR28Oarrow_forwardStraight or with a twist? Account for the different structures of glycogen and cellulose.arrow_forwardPeptide bond properties. How these properties determine the three-dimensional structure of the protein.arrow_forward
- Activity: Write the line structure of each of the following peptide at pH7 and identify how many peptide bond in each number. 1 Alanyl-phenylalanine 2. Lysyl-alanine 3.Phenylalanyl-tyrosyl-leucinearrow_forwardActivity: Write the line structure of each of the following peptide at pH7 and identify how many peptide bond in each number. 1. Glycyl-valyl-serine 2. Threonyl-cysteine 3. Isoleucyl-methionyl-aspartatearrow_forwardDisadvantages of cholesterolarrow_forward
- Protein folding with PDI and Peptidyl-prolyl isomerasearrow_forwardThinking about the complexity of biochemical systems as they relate to the human body and the specificity of DNA, why can we not describe the “average” behavior of a DNA molecule?arrow_forwardBIOMOLECULES - Please answer the questions properly. - Multiple choice 1. Chymotrypsin is a digestive enzyme that breaks down proteins. From the following attributes, which one does not characterize chymotrypsin? A. A thiol nucleophile B. Performs proteolysis C. A tetrahedral intermediate D. A catalytic triad of serine, histidine, and aspartate 2. Evaluate the secondary structure of proteins. Which of the following differentiates alpha structures to those beta structures? ? A. alpha structures are helices and beta structures are pleated sheets B. alpha structures are primary and beta structures are secondary C. alpha structures are parallel and beta structures are antiparallel D. alpha structures are L and beta structures are Darrow_forward
- BIOMOLECULES - Please answer the questions properly. - Multiple choice 1. One of the following statements best describes the attribute of a “protein motif”. Which one is it?* A. a commonly occurring arrangement made up of tertiary structures B. a commonly occurring arrangement made up of multiple secondary structures C. a unique arrangement made up of tertiary structures found only in a single protein D. a family of proteins with similar functions 2. Find out the reason why antiparallel beta sheets are more stable than parallel beta sheets? A. the hydrogen bonding angle is optimized by antiparallel sheets B. there are more covalent interactions between its amino acids C. the antiparallel sheets are composed of more stable amino acids D. the hydrogen bond angle is 150 degreesarrow_forwardresting state of the protein, the Lys 216 Schiff base has pKa = 9.5 and Asp 85 has pKa = 3.5. When the conformational change occurs, the proton that was on the Schiff base moves to Asp 85. This should tell you that one or both of these two pKas changed with the conformational change. How does the pKa of Asp 85 compare with the pKa of the Lys 216 Schiff base after the conformational change?arrow_forwardstructure of cholesterol, define the three regions of the molecule, know that this molecule regulates membrane fluidity, and distinguish between esterified and unesterifiedcholesterolarrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
