
(a)
Interpretation:
The formulas of all the compounds containing the cation Ba+2 and anions I-or N3 - needs to be determined.
Concept introduction:
There are two types of ions:
Cations = These are positively charged formed by losing electrons.
Anions = These are negatively charged ions formed by gaining electrons.
Cations and anions combine to form ionic compounds.
Steps to write chemical formula from ions:
Let’s say there are two ions A+3 and B-2.
- Identify the cations and anions.
Cations are positively charged, and anions are negatively charged.
Here
A+3 = cation
B-2 = anion
- Write both cation and anion together (placing cation first) as:
- Put a value in the subscript of anion which is equal in magnitude with charge of cation and in the subscript of cation, put the value equals to magnitude of charge of anion as:
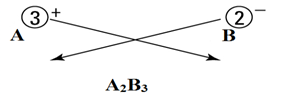
- If the subscript of anions and cations are multiple of a common number, then make it the smallest whole number ratio.
(b)
Interpretation:
The formulas of all the compounds containing the cations Fe+2 or Fe+3 and anion O-2 needs to be determined.
Concept introduction:
There are two types of ions:
Cations = These are positively charged formed by losing electrons.
Anions = These are negatively charged ions formed by gaining electrons.
Cations and anions combine to form ionic compounds.
Steps to write chemical formula from ions:
Let’s say there are two ions A+3 and B-2.
Cations are positively charged, and anions are negatively charged.
Here
A+3 = cation
B-2 = anion
- Write both cation and anion together (placing cation first) as:
- Put a value in the subscript of anion which is equal in magnitude with charge of cation and in the subscript of cation, put the value equals to magnitude of charge of anion as:
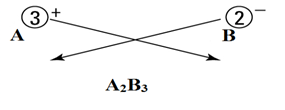
- If the subscript of anions and cations are multiple of a common number, then make it the smallest whole number ratio.
Trending nowThis is a popular solution!

Chapter 2 Solutions
Chemistry: Principles and Reactions
- Name the following ionic compounds: (a) KCN, (b) NaBrO2,(c) Sr1OH22, (d) CoTe, (e) Fe21CO323, ( f ) Cr1NO323?arrow_forwardWhich of the following are ionic, and which are molecular?(a) PF5, (b) NaI, (c) SCl2, (d) Ca1NO322, (e) FeCl3, (f) LaP,(g) CoCO3, (h) N2O4.arrow_forwardCobalt(II) sulfate heptahydrate has pink-colored crystals. When heated carefully, it produces cobalt(II) sulfate monohydrate, which has red crystals. What are the formulas of these hydrates? If 3.548 g of the heptahydrate yields 2.184 g of the monohydrate, how many grams of the anhydrous cobalt(II) sulfate could be obtained?arrow_forward
- A sample of green crystals of nickel(II) sulfate heptahydrate was heated carefully to produce the bluish green nickel(II) sulfate hexahydrate. What are the formulas of the hydrates? If 8.753 g of the heptahydrate produces 8.192 g of the hexahydrate, how many grams of anhydrous nickel(II) sulfate could be obtained?arrow_forwardGive the complete symbol (XZA), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, and (b) a tungsten atom with 110 neutrons.arrow_forwardThe formula of water is If-O. Which of the following is indicated by this formula? Explain your answer. a. The mass of hydrogen is twice that of oxygen in each molecule. b. There are two hydrogen atoms and one oxygen atom per water molecule. c. The mass of oxygen is twice that of hydrogen in each molecule. d. There are two oxygen atoms and one hydrogen atom per water molecule.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





