
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 80QAP
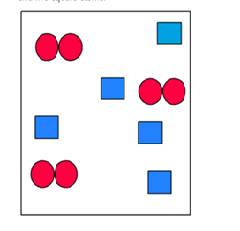
Use the law of conservation of mass to determine which numbered box(es) represent(s) the product mixture after the substances in the unnumbered box undergo a reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compounds are composed two or more elements chemically combined while mixture are composed of substances where th
components can be separnted. In connection with this, where do you compare your family, to a compound or a mixture? Why?
Distinguish between molecular substances and ionic substances in terms of their composition?
During a filtration or distillation experiment, we separate a mixture into its individual components. Do the chemical identities of the components of the mixture change during such a process? Explain.
Chapter 2 Solutions
Chemistry: Principles and Reactions
Ch. 2 - Atomic Theory and Laws State in your own words the...Ch. 2 - State in your own words the law of constant...Ch. 2 - Two basic laws of chemistry are the law of...Ch. 2 - Two basic laws of chemistry are the law of...Ch. 2 - Who discovered the electron? Describe the...Ch. 2 - Who discovered the nucleus? Describe the...Ch. 2 - Selenium is widely sold as a dietary supplement....Ch. 2 - Radon is a radioactive gas that can cause lung...Ch. 2 - How do the isotopes of argon, Ar-36, Ar-38, and...Ch. 2 - Consider two isotopes Fe-54 and Fe-56. (a) Write...
Ch. 2 - Uranium-235 is the isotope of uranium commonly...Ch. 2 - An isotope of americium (Am) with 146 neutrons is...Ch. 2 - Prob. 13QAPCh. 2 - Prob. 14QAPCh. 2 - Prob. 15QAPCh. 2 - See the definition for isobars in Question 15....Ch. 2 - Calculate the mass ratio of a bromine atom to an...Ch. 2 - Arrange the following in the order of increasing...Ch. 2 - Cerium is the most abundant rare earth metal. Pure...Ch. 2 - Consider the three stable isotopes of oxygen with...Ch. 2 - Bromine has two occuring isotopes: 79Br with...Ch. 2 - Rubidium has two naturally occurring isotopes:...Ch. 2 - Strontium has four isotopes with the following...Ch. 2 - Neon is an inert gas with three stable isotopes....Ch. 2 - Naturally occurring silver (Ag) consists of two...Ch. 2 - Copper has two naturally occurring isotopes. Cu-63...Ch. 2 - Silicon (averageatomicmass=28.0855amu) has three...Ch. 2 - Magnesium (averageatomicmass=24.305amu) consists...Ch. 2 - Zinc has four stable isotopes: Zn-64, Zn-66,...Ch. 2 - Chlorine has two isotopes, Cl-35 and Cl-37. Their...Ch. 2 - Lead is a heavy metal that remains in the...Ch. 2 - Silversmiths are warned to limit their exposure to...Ch. 2 - Determine (a) the number of atoms in 0.185 g of...Ch. 2 - For bismuth (Bi), determine (a) the number of...Ch. 2 - The isotope Si-28 has a mass of 27.977 amu. For...Ch. 2 - Myocardial perfusion imaging (MPI) is the latest...Ch. 2 - A cube of sodium has length 1.25 in. How many...Ch. 2 - A cylindrical piece of pure copper (d=8.92g/cm2)...Ch. 2 - Give the symbols for (a) potassium (b) cadmium (c)...Ch. 2 - Prob. 40QAPCh. 2 - Prob. 41QAPCh. 2 - Prob. 42QAPCh. 2 - How many metals are in the following groups? (a)...Ch. 2 - How many nonmetals are in the following periods?...Ch. 2 - Which group in the periodic table (a) has one...Ch. 2 - Which period of the periodic table (a) has no...Ch. 2 - Prob. 47QAPCh. 2 - Prob. 48QAPCh. 2 - Prob. 49QAPCh. 2 - Prob. 50QAPCh. 2 - Prob. 51QAPCh. 2 - Complete the table given below.Ch. 2 - Classify the following compounds as electrolytes...Ch. 2 - Prob. 54QAPCh. 2 - Prob. 55QAPCh. 2 - Prob. 56QAPCh. 2 - Prob. 57QAPCh. 2 - Write the names of the following molecules. (a)...Ch. 2 - Prob. 59QAPCh. 2 - Prob. 60QAPCh. 2 - Prob. 61QAPCh. 2 - Prob. 62QAPCh. 2 - Prob. 63QAPCh. 2 - Prob. 64QAPCh. 2 - Write the names of the following ionic compounds....Ch. 2 - Prob. 66QAPCh. 2 - Complete the following table.Ch. 2 - Complete the following table.Ch. 2 - Prob. 69QAPCh. 2 - Prob. 70QAPCh. 2 - Prob. 71QAPCh. 2 - Prob. 72QAPCh. 2 - Criticize each of the following statements: (a)...Ch. 2 - Which of the following statements is/are always...Ch. 2 - Some brands of salami contain 0.090% sodium...Ch. 2 - Carbon tetrachloride, CCl4, was a popular...Ch. 2 - Prob. 77QAPCh. 2 - Prob. 78QAPCh. 2 - Prob. 79QAPCh. 2 - Use the law of conservation of mass to determine...Ch. 2 - Prob. 81QAPCh. 2 - Prob. 82QAPCh. 2 - Scientists are trying to synthesize elements with...Ch. 2 - Write the nuclear symbol for the element whose...Ch. 2 - Prob. 85QAPCh. 2 - Write the atomic symbol for the element whose ion...Ch. 2 - Prob. 87QAPCh. 2 - Three compounds containing only carbon and...Ch. 2 - Ethane and ethylene are two gases containing only...Ch. 2 - Calculate the average density of a single Al-27...Ch. 2 - Prob. 91QAPCh. 2 - Each time you inhale, you take in about 500 mL...Ch. 2 - Hydrogen gas is prepared in a lab experiment. In...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following table by filling in the blanks in each row. The first row has been completed as an example.arrow_forwardTo which classes of matter—element, compound, and/ormixture—do the following apply: (a) law of mass conservation;(b) law of definite composition; (c) law of multiple proportions?arrow_forwardIdentify all of the true statements regarding elements, compounds, and pure substances. Pure elements can have atoms with 12 and 13 protons, respectively. Pure compounds can have atoms with 12 and 13 protons. If two samples of the same compound are collected, the same ratio of atoms will be observed in both samples. Samples of different compounds will always have different atoms present.arrow_forward
- Which of the following are pure substances? Explain.(a) Calcium chloride, used to melt ice on roads, consists of twoelements, calcium and chlorine, in a fixed mass ratio.(b) Sulfur consists of sulfur atoms combined into octatomicmolecules.(c) Baking powder, a leavening agent, contains 26% to 30%sodium hydrogen carbonate and 30% to 35% calcium dihydro-gen phosphate by mass.(d) Cytosine, a component of DNA, consists of H, C, N, and Oatoms bonded in a specific arrangement.arrow_forwardWhich of the following are pure substances? Explain.(a) Calcium chloride, used to melt ice on roads, consists of twoelements, calcium and chlorine, in a fixed mass ratio.(b) Sulfur consists of sulfur atoms combined into octatomicmolecules.(c) Baking powder, a leavening agent, contains 26% to 30%sodium hydrogen carbonate and 30% to 35% calcium dihydro-gen phosphate by mass.(d) Cytosine, a component of DNA, consists of H, C, N, and Oatoms bonded in a specific arrangement.Classify each substance as an element, com-pound, or mixture, and explain your answersarrow_forwardWhich of the following statements is true about a compound? A compound is always made up of different types of atoms bonded together in proportions that may vary. A compound is always made up of the same type of elements bonded together in fixed proportions. A compound is always made up of different types of atoms bonded together in proportions that are fixed. All of the above.arrow_forward
- Defend or refute the statement • The separation of a mixture may result in the production of an elementarrow_forwardWhat does 1/5 have to do with this equation? You take three compounds, each consisting of two elements (X, Y, and/or Z), and decompose them to their respective ele- ments. To determine the relative masses of X, Y, and Z, you collect and weigh the elements, obtaining the following data: What are the assumptions needed to solve this problem? b. What are the relative masses of X, Y, and Z?c. What are the chemical formulas of the three compounds? d. If you decompose 21 g of compound XY, how much of each element is present?arrow_forwardWhich of the following is a property of carbon atoms? im not sure which one because arent carbon atoms found in all living things? idk which one of these it issss becuase carbon reactions with some stuff too so idk They are chemically inert. They readily give up two electrons. They are found in all living things.arrow_forward
- In 1808 John Dalton published his atomic theory which was quickly adopted. The five tenets of Dalton's theory are An element is composed of tiny indivisible, indestructible particles called atoms. All the atoms of an element are identical and have the same properties. Atoms of different elements combine to form compounds. Compounds contain atoms in ratios of small whole numbers. Atoms can combine in more than one ratio to form different compounds. Although all of the statements were valid at the time, several have been modified as new discoveries were made. Which of these tenets have been modified? What caused the modification?arrow_forwardWhich of the following statements are true of chemical reactions? a. The number and types of atoms are the same in the products and the reactants. b. Different isotopes of the same element behave similarly. c. New elements are often formed. d. Only the valence electrons are involved.arrow_forwardWater and sodium chloride are both considered substances as well as compounds, but water exists as molecules while sodium chloride does not. (i) Explain this difference in the behavior of water and sodium chloride. As part of your explanation, (ii) define the terms substance, molecule, and compound.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY