
(a)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
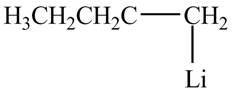
Figure 1
Explanation of Solution
The halogen group of
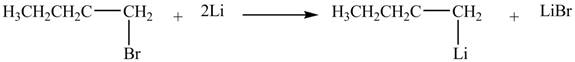
Figure 2
The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
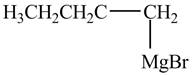
Figure 3
Explanation of Solution
The halogen group of alkyl halide forms bond with
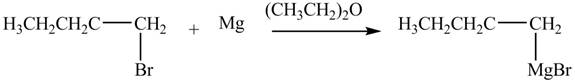
Figure 4
The product formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 5
Explanation of Solution
The halogen group of alkyl halide is replaced by
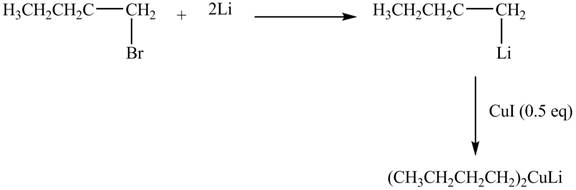
Figure 6
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 7
Explanation of Solution
The product formed by the reaction of
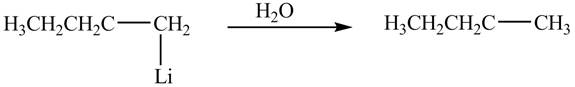
Figure 8
The product formed by the treatment of
(e)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 9
Explanation of Solution
The product formed by the reaction of
![]()
Figure 10
The product formed by the treatment of
(f)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 11
Explanation of Solution
The product formed by the reaction of
![]()
Figure 12
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - Access (Custom)
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardIdentify the reagent e, f, g and harrow_forward29. Draw the most stable enol tautomer for each and provide one brief reason for each explaining why it is more stable.arrow_forward
- Draw the product formed when pentanal (CH3CH2CH2CH2CHO) is treatedwith following reagent. With some reagents, no reaction occurs. [1] CH3C≡CLi; [2] H2Oarrow_forwardDraw the products formed when CH3CH2C=C Na+ reacts with each compound. a. CH3CH2CH2Br b.(CH3)2CHCH2CH2Cl c.(CH3CH2)3CCl d.BrCH2CH2CH2CH2OH e. ethylene oxide followed by H2O f.propene oxide followed by H2Oarrow_forwardillustrate how each reactant appeared prior to the reaction and how the product appeared after the reaction. 2Na+2H₂O --> 2NaOH+H₂arrow_forward
- #20 B Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HCl.arrow_forwardDraw the product formed when pentanal (CH3CH2CH2CH2CHO) is treated with each reagent. With some reagents, no reaction occurs. [1] (CH3)2CuLi; [2] H2Oarrow_forwardLabel the α and β carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K+−OC(CH3)3.arrow_forward
- Give the IUPAC name (including any E,Z designation) for each unsaturated aldehyde. Neral is obtained from lemon grass, and cucumber aldehyde (Problem 1.30) contributes to the aroma of a fresh mango.arrow_forwardDraw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
